3-三氟甲基苄硫醇 | 25697-55-6
中文名称
3-三氟甲基苄硫醇
中文别名
3-(三氟甲基)苯甲硫醇;3-(三氟甲基)苄基硫醇
英文名称
m-trifluoromethylbenzyl mercaptan
英文别名
(3-(trifluoromethyl)phenyl)methanethiol;3-(trifluoromethyl)benzyl thiol;[3-(Trifluoromethyl)phenyl]methanethiol
CAS
25697-55-6
化学式
C8H7F3S
mdl
MFCD00042435
分子量
192.205
InChiKey
CQIQWIMXCPTQPJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:79 °C
-
密度:1.253±0.06 g/cm3(Predicted)
-
闪点:75-77°C/5mm
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。避免接触氧化物和空气。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S23,S36/37
-
危险类别码:R20/21/22
-
危险品运输编号:2810
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
SDS
| Name: | [3-(Trifluoromethyl)phenyl]methanethiol tech Material Safety Data Sheet |
| Synonym: | |
| CAS: | 25697-55-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 25697-55-6 | [3-(Trifluoromethyl)phenyl]methanethio | unlisted |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.Moisture sensitive.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. May cause respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Combustible liquid.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Keep away from sources of ignition. Store in a cool, dry place.
Store in a tightly closed container. Store under nitrogen.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 25697-55-6: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless - pale yellow
Odor: stench
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 75 - 77 deg C @5mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H7F3S
Molecular Weight: 192
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Oxidizing agents, bases, amines.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide, fluorine, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 25697-55-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
[3-(Trifluoromethyl)phenyl]methanethiol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 25697-55-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 25697-55-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 25697-55-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— [3-(Trifluoromethyl)phenyl]methyl carbamimidothioate 792843-57-3 C9H9F3N2S 234.245 1-氯甲基-3-三氟甲基苯 3-Trifluoromethylbenzyl chloride 705-29-3 C8H6ClF3 194.584 3-(三氟甲基)溴苄 1-bromomethyl-3-trifluoromethylbenzene 402-23-3 C8H6BrF3 239.035 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-[(4-chlorobutyl)thiomethyl]-3-trifluoromethylbenzene 124880-18-8 C12H14ClF3S 282.757 3-三氟甲基苯甲醛 3-Trifluoromethylbenzaldehyde 454-89-7 C8H5F3O 174.122 —— 1-(4-Chlorobutylsulfinylmethyl)-3-(trifluoromethyl)benzene 124880-14-4 C12H14ClF3OS 298.757 —— hexakis-(3-trifluoromethylbenzylthiomethyl)benzene 75155-59-8 C60H48F18S6 1303.41
反应信息
-
作为反应物:描述:3-三氟甲基苄硫醇 在 dipotassium peroxodisulfate 、 tetrakis(pyridine)silver(II) peroxodisulfate 、 氧气 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 23.0 ℃ 、101.33 kPa 条件下, 以75%的产率得到3-三氟甲基苯甲醛参考文献:名称:可见光介导的银(II)配合物实现的苄基硫醇的氧化CS键裂解。摘要:使用单线态氧进行C–S键的氧化裂解反应具有挑战性,因为其性质不可控。我们已经开发了一种使用银(II)-配体配合物进行单线态氧介导的选择性CS键断裂反应的新方法。可见光诱导的银催化作用使苄基硫醇能够被控制地氧化裂解,从而提供羰基化合物,例如醛或酮,它们是重要的合成组分。DOI:10.1021/acs.orglett.0c01399
-
作为产物:描述:参考文献:名称:反式(+-)-N-甲基-2-(3-吡啶基)-2-四氢硫代吡喃碳硫酰胺1-氧化物(RP 49356)及其类似物的合成和生物活性:一类新型的钾通道开放剂。摘要:报道了反式-(+-)-N-甲基-2-(3-吡啶基)-2-四氢硫代吡喃氨基甲磺酰胺+++ e 1-氧化物(8a,RP 49356)和类似物的合成和生物活性。这些化合物构成K(+)通道开放剂的新结构类。讨论了吡啶基,硫代酰胺和硫杂环上的变化对体外K(+)通道开放反应性的影响。为了活性,优选3-吡啶基或3-喹啉基,小的N-烷基硫代酰胺官能团和氧化亚砜环,其中亚砜与硫代酰胺具有反式关系。选择的化合物在降压麻醉的大鼠中静脉内测试降压作用,其活性反映了其体外K(+)通道的开放活性。DOI:10.1021/jm00098a004
文献信息
-
Inhibitors of c-Jun N-terminal kinases申请人:Liu Gang公开号:US20060173050A1公开(公告)日:2006-08-03The present invention relates to compounds that are inhibitors of c-jun N-terminal kinase 1, 2, or 3 (JNK1, JNK2, or JNK3), compositions containing the compounds and the use of the compounds in the prevention or treatment of disorders regulated by the activation of JNK1, JNK2 and JNK3.本发明涉及作为c-jun N-末端激酶1、2或3(JNK1、JNK2或JNK3)抑制剂的化合物,包含这些化合物的组合物以及这些化合物在预防或治疗由JNK1、JNK2和JNK3激活调控的疾病中的用途。
-
[EN] MODULATORS OF HSD17B13 AND METHODS OF USE THEREOF<br/>[FR] MODULATEURS DE HSD17B13 ET LEURS PROCÉDÉS D'UTILISATION申请人:REGENERON PHARMA公开号:WO2021003295A1公开(公告)日:2021-01-07The disclosure relates to compounds and pharmaceutical compositions capable of modulating the hydroxysteroid 17-beta dehydrogenase (HSD17B) family member proteins including inhibiting the HSD17B member proteins, e.g. HSD17B13. The disclosure further relates to methods of treating liver diseases, disorders, or conditions with the compounds and pharmaceutical compositions disclosed herein, in which the HSD17B family member protein plays a role.
-
Conversion of thiols into sulfonyl halogenides under aerobic and metal-free conditions作者:Marjan Jereb、Luka HribernikDOI:10.1039/c7gc00556c日期:——a redox-catalytic cycle. Sulfonyl chlorides and bromides were isolated without extraction and “filtered” over a short pad of silica gel; the use of solvents was greatly reduced in comparison with traditional isolation and purification. A “one-pot” protocol for the conversion of thiol into sulfonamide is also demonstrated. Scale-up experiments on the preparation of sulfonyl chloride and bromide are
-
Base-controlled Fe(Pc)-catalyzed aerobic oxidation of thiols for the synthesis of S–S and S–P(O) bonds作者:Hai Huang、Jeffrey Ash、Jun Yong KangDOI:10.1039/c8ob00908b日期:——disulfides has been developed under mild reaction conditions. In addition, an aerobic oxidative cross-dehydrogenative coupling (CDC) reaction of thiols with P(O)–H compounds (H-phosphonates and H-phosphine oxide) for the formation of S–P(O) bonds has been demonstrated under the Fe(Pc) catalysis system with a base additive. Control experiments revealed that the use of a base (DIPA) in this system controls
-
Dihydropyrancarboxamides and uses thereof申请人:——公开号:US20040059138A1公开(公告)日:2004-03-25The present invention provides novel dihydropyrancarboxamide compounds of formula (I): 1 and collections of these compounds, and provides methods for the synthesis of these compounds; wherein R 1 -R 6 are as defined herein. Additionally, the present invention provides pharmaceutical compositions and methods for treating disorders such as proliferative diseases, and cancer, to name a few.本发明提供了式(I)的新颖二氢吡喃羧酰胺化合物: 1 以及这些化合物的集合,并提供了合成这些化合物的方法;其中R 1 -R 6 如本文所定义。此外,本发明提供了用于治疗增殖性疾病和癌症等疾病的药物组合物和方法。
表征谱图
-
氢谱1HNMR
-
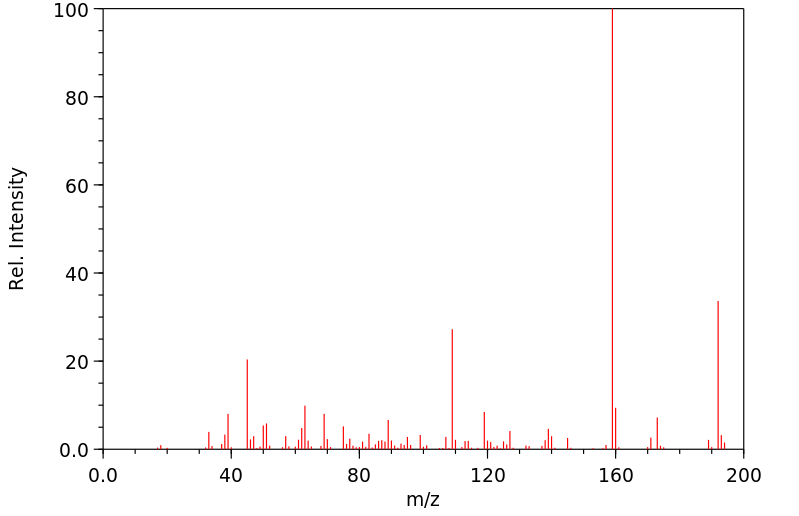
质谱MS
-
碳谱13CNMR
-
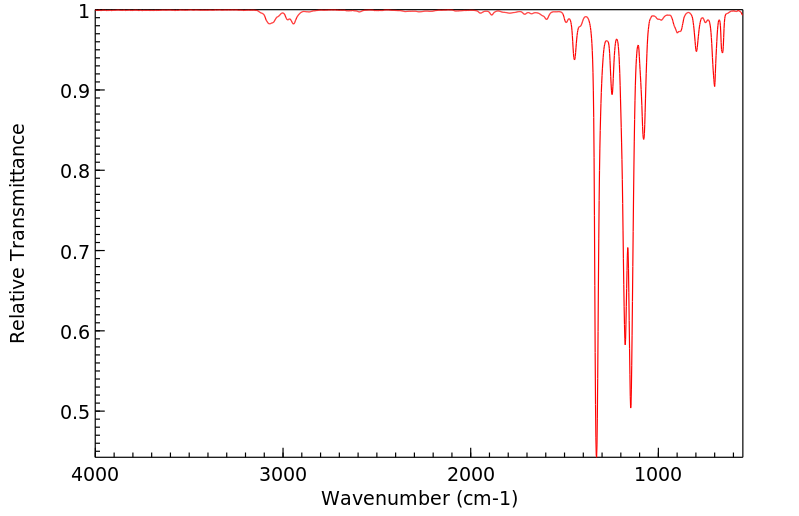
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫