4,4'-双乙酰联苯 | 32604-29-8
中文名称
4,4'-双乙酰联苯
中文别名
4,4'-二乙酰氧基联苯;4,4'-双乙酰氧基联苯;4,4’-双乙酰氧基联苯
英文名称
4,4'-diacetoxybiphenyl
英文别名
[4-(4-acetyloxyphenyl)phenyl] acetate
CAS
32604-29-8
化学式
C16H14O4
mdl
MFCD00035973
分子量
270.285
InChiKey
RQMBBMQDXFZFCC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160-161°C
-
沸点:398.8±35.0 °C(Predicted)
-
密度:1.175±0.06 g/cm3(Predicted)
-
溶解度:溶于丙酮
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2915390090
-
储存条件:请将贮藏器密封保存,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
4,4'-二乙酰氧基联苯 修改号码:6
模块 1. 化学品
产品名称: 4,4'-Diacetoxybiphenyl
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二乙酰氧基联苯
百分比: ....
CAS编码: 32604-29-8
分子式: C16H14O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
4,4'-二乙酰氧基联苯 修改号码:6
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
164°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 丙酮
4,4'-二乙酰氧基联苯 修改号码:6
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
4,4'-二乙酰氧基联苯 修改号码:6
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4,4'-Diacetoxybiphenyl
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二乙酰氧基联苯
百分比: ....
CAS编码: 32604-29-8
分子式: C16H14O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
4,4'-二乙酰氧基联苯 修改号码:6
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
164°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 丙酮
4,4'-二乙酰氧基联苯 修改号码:6
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
4,4'-二乙酰氧基联苯 修改号码:6
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,4-二甲氧基联苯 4,4'-Dimethoxybiphenyl 2132-80-1 C14H14O2 214.264 4,4'-二羟基联苯 4,4'-Dihydroxybiphenyl 92-88-6 C12H10O2 186.21 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4,4'-二羟基联苯 4,4'-Dihydroxybiphenyl 92-88-6 C12H10O2 186.21
反应信息
-
作为反应物:描述:4,4'-双乙酰联苯 在 aluminum (III) chloride 、 盐酸 作用下, 以 氯苯 、 水 为溶剂, 反应 8.0h, 以72%的产率得到4,4'-Dihydroxy-3,3'-diacetyl-biphenyl参考文献:名称:Synthesis and antifungal activities of natural and synthetic biflavonoids摘要:The synthesis of some natural and synthetic biflavonoids was performed in good overall yields starting from readily available materials via high yielding aldol and Ullmann condensations. Some of these compounds, especially bichalcones, display an interesting activity against fungi, higher than that of the corresponding monomers. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2011.04.010
-
作为产物:描述:参考文献:名称:Schmidt; Schultz, Justus Liebigs Annalen der Chemie, 1881, vol. 207, p. 333摘要:DOI:
文献信息
-
Guest-Encapsulation Properties of a Self-Assembled Capsule by Dynamic Boronic Ester Bonds作者:Naoki Nishimura、Kenji Yoza、Kenji KobayashiDOI:10.1021/ja9084918日期:2010.1.20selective recognition event, wherein the guest substituents are oriented to both aromatic cavity ends of 3a, as confirmed by a (1)H NMR study and X-ray crystallographic analysis. Capsule 3a showed a significant solvent effect on guest encapsulation. The association constant (K(a)) of 3a with guests in C(6)D(6) was much greater than that in CDCl(3) (450-48,000-fold). The encapsulation of guests within两分子四(二羟基硼基)-空腔和 1a 作为芳香腔,四分子 1,2-双(3,4-二羟基苯基)乙烷 2 作为赤道连接体通过形成八动态硼酸酯自组装成胶囊 3a CDCl(3) 或 C(6)D(6) 中的键。胶囊 3a 在高度选择性识别事件中封装了一个客体分子,例如 4,4'-二取代-联苯和 2,6-二取代-蒽衍生物,其中客体取代基定向到 3a 的两个芳香腔末端,如证实通过 (1)H NMR 研究和 X 射线晶体学分析。胶囊 3a 显示出对客体封装的显着溶剂效应。3a 与客人在 C(6)D(6) 中的关联常数 (K(a)) 比在 CDCl(3) 中的要大得多(450-48,000 倍)。C(6)D(6) 中 3a 内客体的封装是由焓驱动的,而CDCl(3) 中的趋向于同时受焓和熵驱动。热力学研究表明,CDCl(3) 中较小的 K(a) 值源于 CDCl(3) 作为 3a 的竞争客体分子的特性,而不是源于
-
Reductive Homocoupling of Organohalides Using Nickel(II) Chloride and Samarium Metal作者:Yongjun Liu、Shuhuan Xiao、Yan Qi、Feng DuDOI:10.1002/asia.201601712日期:2017.3.16(benzyl, aryl, heterocyclic, alkenyl and alkyl halides), α‐haloacetophenones, and phenyl organosulfonates were tolerated, and the reaction afforded coupling products with high efficiency. Excellent chemoselectivity was exhibited between halides and other groups, such as −COOH, −NO2, halogen, heterocyclic ring, ester, and ketone groups. The stereoselectivity suggested that the reaction mechanism might
-
Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides作者:Daniel A. Everson、Brittany A. Jones、Daniel J. WeixDOI:10.1021/ja301769r日期:2012.4.11general method is presented for the synthesis of alkylated arenes by the chemoselective combination of two electrophilic carbons. Under the optimized conditions, a variety of aryl and vinyl bromides are reductively coupled with alkyl bromides in high yields. Under similar conditions, activated aryl chlorides can also be coupled with bromoalkanes. The protocols are highly functional-group tolerant (−OH,提出了通过两个亲电碳的化学选择性组合合成烷基化芳烃的一般方法。在优化的条件下,各种芳基和乙烯基溴化物以高产率与烷基溴化物还原偶联。在类似条件下,活化的芳基氯也可以与溴代烷烃偶联。该协议具有高度的官能团耐受性(-OH、-NHTs、-OAc、-OTs、-OTf、-COMe、-NHBoc、-NHCbz、-CN、-SO2Me),并且反应在台式上组装,无需排除空气或湿气的特殊预防措施。该反应显示出与传统交叉偶联反应不同的化学选择性,例如铃木-宫浦反应、斯蒂尔反应和日山-丹麦反应。带有亲电和亲核碳的底物导致亲电碳 (R-X) 处的选择性偶联,并且在亲核碳 (R-[M]) 处不发生有机硼 (-Bpin)、有机锡 (-SnMe3) 和有机硅的反应(-SiMe2OH) 含有机卤化物 (X–R–[M])。Hammett 研究表明,σ 和 σ(-) 参数与取代芳基溴化物与溴代烷烃的相对反应速率呈线性相关。这些相关性
-
Recyclable and reusable Pd(OAc)<sub>2</sub>/PPh<sub>3</sub>/PEG-2000 system for homocoupling reaction of arylboronic acids under air without base作者:Jianhui Xia、Mingzhu Cheng、Qiurong Chen、Mingzhong CaiDOI:10.1002/aoc.3254日期:2015.2A stable and efficient Pd(OAc)2/PPh3/PEG‐2000 catalytic system for homocoupling of arylboronic acids has been developed. In the presence of Pd(OAc)2 and PPh3, the homocoupling reaction of arylboronic acids was carried out smoothly in PEG‐2000 at 70 °C under air without base to afford a variety of symmetric biaryls in good to excellent yields. The isolation of the products was readily performed by extraction
-
Phosphine ligand triggered oxidative decarbonylative homocoupling of aromatic aldehydes: selectively generating biaryls and diarylketones作者:Luo Yang、Tieqiang Zeng、Qi Shuai、Xiangyu Guo、Chao-Jun LiDOI:10.1039/c0cc04921b日期:——A novel rhodium-catalyzed oxidative decarbonylative homocoupling of aromatic aldehydes to generate biaryls and diarylketones selectively and efficiently, triggered by the choice of different phosphine ligands.
表征谱图
-
氢谱1HNMR
-
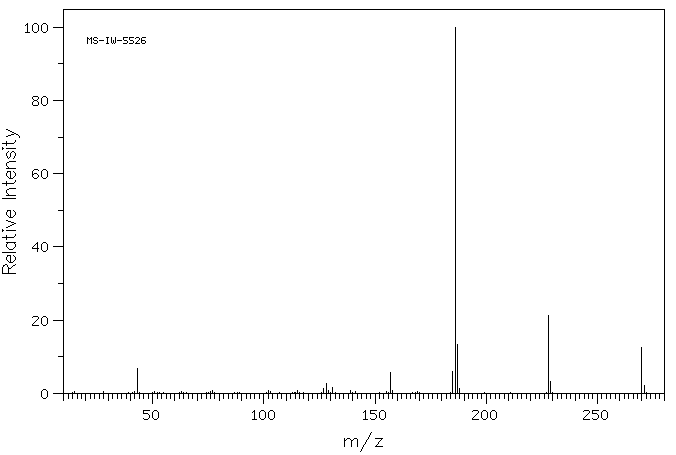
质谱MS
-
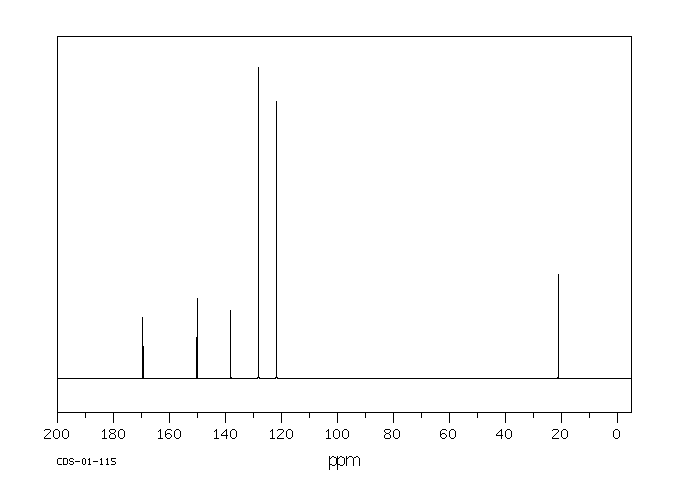
碳谱13CNMR
-
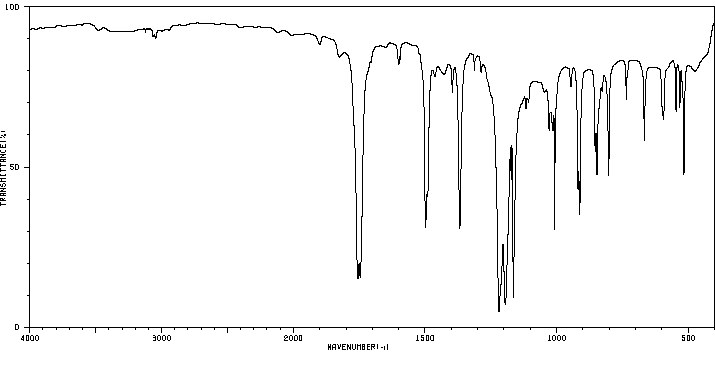
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫