4-乙基苯硼酸频哪醇酯 | 1075719-87-7
中文名称
4-乙基苯硼酸频哪醇酯
中文别名
4-乙基苯硼酸频呐醇酯
英文名称
2-(4-ethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
英文别名
4-ethylphenylboronic acid pinacol ester
CAS
1075719-87-7
化学式
C14H21BO2
mdl
——
分子量
232.131
InChiKey
RWANCHDMVNWYHW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:313.0±21.0 °C(Predicted)
-
密度:0.97±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.55
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温且干燥
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(1-羟基乙基)苯硼酸频哪醇酯 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethanol 1173922-30-9 C14H21BO3 248.13 4-乙酰苯基硼酸,醇酯 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethanone 171364-81-1 C14H19BO3 246.114
反应信息
-
作为反应物:描述:参考文献:名称:5-苯基嘧啶-2-硫醇 铜(i)配合物显示出独特的光学性能和高可见光定向催化性能†摘要:用等摩尔的CuBr 5-苯基嘧啶-2-硫醇(5- phpymtH)的溶剂热反应得到一个六核簇[铜6(μ 3 -5-phpymt)6 ](1)用四核副产品沿[{(CU 2 BR)(μ-5-phpymtH)}(μ 3 -5-phpymt)] 2(2)。的二维(2D)聚合物[铜4(μ 5 -5- phpymt)2(μ-溴)2 ] Ñ(3)由5- phpymtH具有两个当量的反应中分离。的CuBr。5-phpymtH与一或四个当量的类似反应。的CuI产生了一个四核簇[{Cu 2(μ-5-phpymtH)(μ-5-phpymt)}(μ 3 -I)] 2(4)和一个二维聚合物[铜6我2(μ 4 -I)2(μ 4 -5- phpymt)2 ] n(5)。化合物1具有水轮状的六聚体结构。化合物2具有H形四聚体结构。化合物3具有一个二维网络,其中唯一1D [铜8(μ-溴)2(μ 5 -5- phpymt)4DOI:10.1039/c6dt03721f
-
作为产物:描述:参考文献:名称:Preparation and reactions of 4-iodobutyl pinacolborate. Synthesis of substituted alkyl and aryl pinacolboronates via 4-iodobutyl pinacolborate utilizing tetrahydrofuran as the leaving group摘要:Iodine reacts with 4,4,5,5-tetramethyl-1,3,2-dioxaborolane (HBpin), under ambient reaction conditions in THF, to form the iodoalkylborate species 2-(4-iodobutoxy)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (4-IboxBpin). Apparently, one-half equivalent of 12 reacts with HBpin to form IBpin in pentanes, which in turn cleaves THF to form the 4-IboxBpin. Alkyl and aryl Grignard reagents, prepared under Barbier conditions, then react with 4-IboxBpin to form the corresponding alkyl and aryl pinacolboronates while reforming and liberating THF as the leaving group. Published by Elsevier Ltd.DOI:10.1016/j.tetlet.2014.12.033
文献信息
-
An Opportunity for Mg-Catalyzed Grignard-Type Reactions: Direct Coupling of Benzylic Halides with Pinacolborane with 10 mol % of Magnesium作者:Christine Pintaric、Sandra Olivero、Yves Gimbert、Pierre Y. Chavant、Elisabet DuñachDOI:10.1021/ja1052973日期:2010.9.1Mg in catalytic amounts as the only metal permits the reductive coupling between benzyl halides and pinacolborane. HBpin acts both as an electrophile and as a reducing agent to regenerate an organomagnesium species in situ. An hydride oxidation mechanism is proposed on the basis of DFT calculations.
-
PROCESS FOR PREPARING BORONIC ACIDS AND ESTERS IN THE PRESENCE OF MAGNESIUM METAL申请人:Dunach Isabel公开号:US20110282090A1公开(公告)日:2011-11-17The present invention relates to the process for preparing a boronic acid or ester chemically, in which an aromatic compound is reacted with a boronating agent, in the presence of magnesium metal (Mg 0 ). The invention also relates to the boronic acids or esters that can be obtained by means of this process and to the use thereof for example as a synthesis intermediate, in the Suzuki reaction, in the pharmaceutical or alternatively electronics field.
-
NOVEL COELENTERAZINE COMPOUNDS AND USES THEREOF申请人:KEIO UNIVERSITY公开号:US20180273539A1公开(公告)日:2018-09-27There is provided a series of coelenterazine (CTZ) derivatives as a substrate with high luminescence intensity, which is optimal for maximum luminescence wavelengths at both 400 nm (blue-shifted RLuc luminescence system) and 500 nm (ALuc luminescence system) for bioassays which is more sensitive than known techniques. The novel CTZ derivatives are compounds in which a specific position(s) of the CTZ is/are substituted with a specific substituent(s) as shown, for example, by the Formula [I], and has a higher luminescence intensity than known CTZ derivatives in blue-shifted RLuc luminescence system or ALuc luminescence system.
-
<scp>Pd‐Catalyzed Site‐Selective</scp> Borylation of Simple Arenes <i>via</i> Thianthrenation <sup>†</sup>作者:Xiao‐Yue Chen、Yu‐Hao Huang、Jian Zhou、Peng WangDOI:10.1002/cjoc.202000212日期:2020.11Site‐selective borylation of simple arenes was realized in one pot via an electrophilic thianthrenation/Pd‐catalyzed borylation sequence. The key to achieve this operatically simple process is the use of Pd catalysis, which could tolerate the solvent and acidic conditions used in the thianthrenation step. This protocol features mild conditions, broad functional group tolerance, and simple manipulations
-
Catalytic Boration of Alkyl Halides with Borane without Hydrodehalogenation Enabled by Titanium Catalyst作者:Xianjin Wang、Penglei Cui、Chungu Xia、Lipeng WuDOI:10.1002/anie.202100569日期:2021.5.25titanium‐catalyzed boration of alkyl (pseudo)halides (alkyl‐X, X=I, Br, Cl, OMs) with borane (HBpin, HBcat) is reported. The use of titanium catalyst can successfully suppress the undesired hydrodehalogenation products that prevail using other transition‐metal catalysts. A series of synthetically useful alkyl boronate esters are readily obtained from various (primary, secondary, and tertiary) alkyl electrophiles,
表征谱图
-
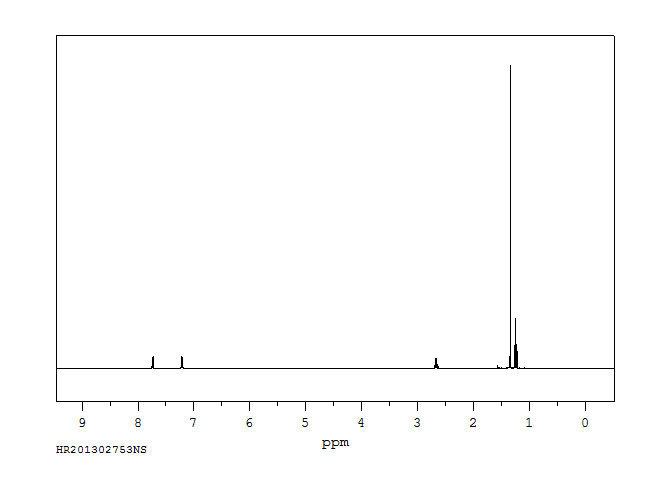
氢谱1HNMR
-
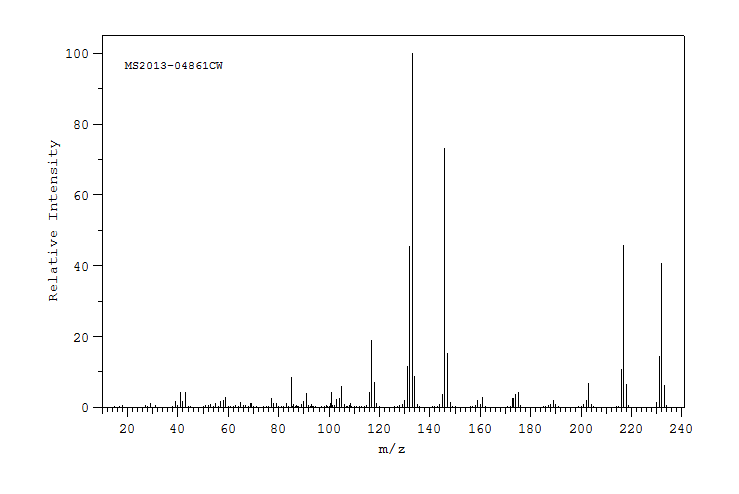
质谱MS
-
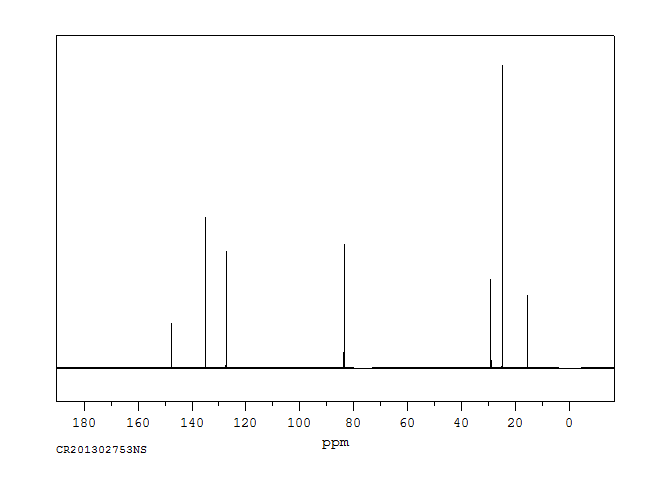
碳谱13CNMR
-
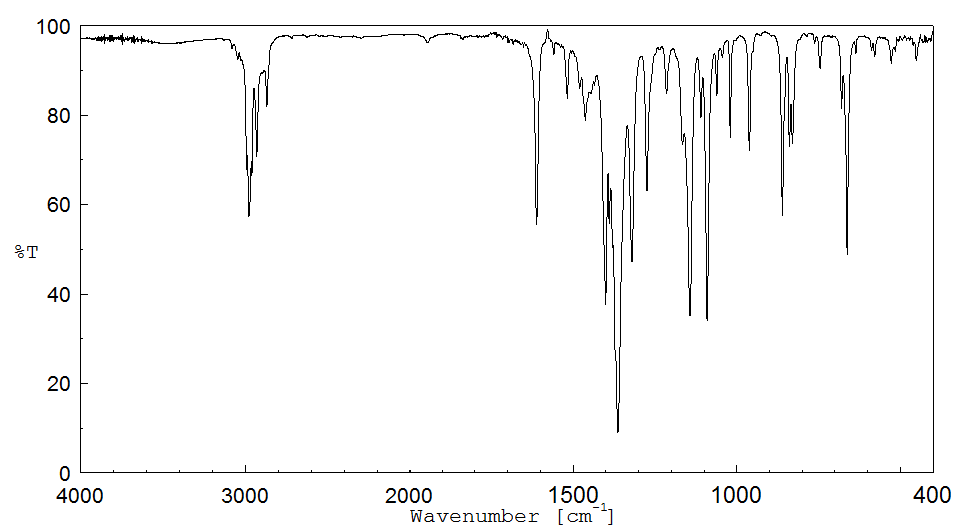
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫