4-氨基-3,5-二碘苯甲酸 | 2122-61-4
中文名称
4-氨基-3,5-二碘苯甲酸
中文别名
——
英文名称
4-amino-3,5-diiodobenzoic acid
英文别名
——
CAS
2122-61-4
化学式
C7H5I2NO2
mdl
MFCD00007684
分子量
388.931
InChiKey
WXTVPMWCUMEVSZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>300 °C(lit.)
-
沸点:448.7±45.0 °C(Predicted)
-
密度:2.3815 (estimate)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:63.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36/37/39,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2922499990
-
RTECS号:DG2310000
-
储存条件:储存地点应远离氧化剂,并确保容器密封,存放在干燥、阴凉处。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氨基-3,5-二碘苯甲酸乙酯 ethyl 4-amino-3,5-diiodobenzoate 5400-81-7 C9H9I2NO2 416.985 4-乙酰氨基-3-碘苯甲酸 4-acetylamino-3-iodo-benzoic acid 190071-24-0 C9H8INO3 305.072 对氨基苯甲酸 4-amino-benzoic acid 150-13-0 C7H7NO2 137.138 3-碘-4-甲基苯胺 2-iodo-4-methylaniline 29289-13-2 C7H8IN 233.052 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氨基-3-碘苯甲酸 4-amino-3-iodobenzoic acid 2122-63-6 C7H6INO2 263.035 甲基4-氨基-3,5-二碘苯甲酸酯 methyl 4-amino-3,5-diiodobenzoate 131755-87-8 C8H7I2NO2 402.958 4-氨基-3,5-二碘苯甲酸乙酯 ethyl 4-amino-3,5-diiodobenzoate 5400-81-7 C9H9I2NO2 416.985 3,5-二碘苯甲酸 3,5-diiodobenzoic acid 19094-48-5 C7H4I2O2 373.917 甲基3,5-二碘苯甲酸酯 methyl 3,5-diiodobenzoate 14266-19-4 C8H6I2O2 387.943 4-羟基-3,5-二碘苯甲酸 3,5-diiodo-4-hydroxybenzoic acid 618-76-8 C7H4I2O3 389.916
反应信息
-
作为反应物:描述:参考文献:名称:Wheeler; Liddle, American Chemical Journal, 1909, vol. 42, p. 457摘要:DOI:
-
作为产物:描述:4-氨基-3,5-二碘苯甲酸乙酯 在 氢氧化钾 作用下, 反应 1.0h, 以98%的产率得到4-氨基-3,5-二碘苯甲酸参考文献:名称:碘氨基戊定和相关化合物:对组胺H2受体具有高亲和力和选择性的新型配体。摘要:描述了一种新型的具有N-氰基-N'-[ω-[3-(1-(哌啶基甲基)苯氧基]烷基]胍部分结构的组胺H2拮抗剂的合成和生物学评估,作为广泛研究计划的一部分,以寻找用于开发具有高H2亲和力和比活性的新型放射性配体的模型化合物。高的受体亲和力是通过一个附加的(取代的)芳环实现的,该芳环通过碳链间隔基和一个胺,羧酰胺,酯或磺酰胺键(“极性基团”)与第三胍N连接。在针对豚鼠心房和回肠以及大鼠主动脉和尾动脉的H2拮抗活性和其他药理作用(例如H1抗组胺药,抗毒蕈碱,抗肾上腺素(α1,β1),5-HT2阻断活性)的功能研究中,该化合物被证明是高效和选择性的组胺H2受体拮抗剂。H2拮抗活性主要取决于N'-烷基链(链A)和N“-间隔基(链B)的长度。具有C3链A和C2链B的化合物最有效。一类物质,即羧酰胺系列,在芳香环上具有广泛的取代基,其中包括碘,氨基和叠氮基,这些化合物在分离的豚鼠右心房中的效能比西咪替丁DOI:10.1021/jm00090a013
文献信息
-
Synthesis, Spectral and Antimicrobial Studies on some Newer Imidazole Analogs作者:Rajiv DahiyaDOI:10.3797/scipharm.0803-04日期:——A novel series of 3,5-diiodo-4-(2-methyl-1H-imidazol-5-yl)benzoic acid analogs of amino acids, dipeptides and tripeptides was synthesized by using dicyclohexylcarbodiimide and diisopropylcarbodiimide (DCC/DIPC) as coupling agents and triethylamine (TEA) as base. Structures of all the newly synthesized compounds were confirmed by elemental analysis and IR, 1H NMR, 13C NMR and mass spectral data. Imidazolopeptides were investigated for their antimicrobial activity and some of newly synthesized compounds 2c, 2d, 2h and their hydrolyzed analogs 3b, 3d exhibited potent bioactivity against pathogenic fungi Candida albicans and dermatophytes Trichophyton mentagrophytes and Microsporum audouinii with MIC values of 12.5-6 μg/ml, as compared to the reference drug - griseofulvin. In addition, moderate activity against gram negative bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae was also observed for synthesized imidazolopeptides.通过使用二环己基碳二亚胺和二异丙基碳二亚胺(DCC/DIPC)作为偶联剂以及三乙胺(TEA)作为碱,合成了一系列新颖的3,5-二碘-4-(2-甲基-1H-咪唑-5-基)苯甲酸氨基酸、二肽和三肽类似物。所有新合成化合物的结构通过元素分析和红外、1H核磁共振、13C核磁共振及质谱数据得到确认。研究了这些咪唑肽的抗菌活性,其中一些新合成的化合物2c、2d、2h及其水解类似物3b、3d显示出对致病真菌白色念珠菌和皮肤癣菌毛癣菌、奥杜安小孢子菌具有强大的生物活性,其最低抑制浓度(MIC)值为12.5-6 μg/ml,相较于参考药物灰黄霉素。此外,合成的咪唑肽还表现出对革兰氏阴性细菌铜绿假单胞菌和肺炎克雷伯菌的中等活性。
-
[EN] PHOTOALIGNING MATERIAL<br/>[FR] MATÉRIAU DE PHOTOALIGNEMENT申请人:ROLIC AG公开号:WO2013017467A1公开(公告)日:2013-02-07The present invention relates to a copolymer for the photoalignment of liquid crystals comprising a photoreactive group as given below in formula (I), compositions thereof, and its use for optical and electro optical devices, especially liquid crystal devices (LCDs).本发明涉及一种共聚物,用于液晶的光调向,包括如下所示的具有光反应性基团的化合物(I)的公式,以及其组合物,以及其在光学和电光设备,特别是液晶设备(LCD)中的使用。
-
Convenient and Efficient Method for the Iodination of Aromatic Amines by Pyridinium Iodochloride作者:Sandeep V. Khansole、Subhash B. Junne、Mudassar A. Sayyed、Yeshwant B. VibhuteDOI:10.1080/00397910801989659日期:2008.5Abstract A simple and efficient method for the iodination of aromatic amines using pyridinium iodochloride (PyICl) in methanol as solvent is reported. Mild reaction conditions, short reaction time, and good to excellent yields of the product are the noteworthy advantages of the method. Pyridinium iodochloride is an efficient solid iodinating reagent and can be handled safely.
-
PHOTOREACTIVE COMPOUNDS申请人:Lincker Frederic公开号:US20140192305A1公开(公告)日:2014-07-10The present invention relates to photoreactive compounds that are particularly useful in materials for the alignment of liquid crystals.本发明涉及光反应性化合物,特别适用于液晶对准材料的制作。
-
A Strategy for the Assembly of Multiple Porphyrin Arrays Based on the Coordination Chemistry of Ru-Centered Porphyrin Pentamers作者:Chi Ching Mak、Nick Bampos、Scott L. Darling、Marco Montalti、Luca Prodi、Jeremy K. M. SandersDOI:10.1021/jo001564o日期:2001.6.1An approach which employs pentameric porphyrin arrays as building blocks toward larger porphyrin arrays is described. Two flexible, and one relatively rigid, Ru-centered porphyrin pentamers (1-3) were synthesized and fully characterized. Their potential as building blocks toward larger porphyrin arrays has been studied via their coordination chemistry using bidentate and tetradentate ligands. DABCO描述了一种采用五聚体卟啉阵列作为朝向较大的卟啉阵列的结构单元的方法。合成了两种挠性和一种相对刚性的以Ru为中心的卟啉五聚体(1-3),并进行了充分表征。通过使用二齿和四齿配体的配位化学研究了它们作为更大的卟啉阵列构建基的潜力。DABCO(二氮杂双环[2.2.2]辛烷)可以结合两个单体卟啉,但发现其太小而无法完全形成10个卟啉阵列。另一方面,用1或2滴定较大的桥联二吡啶基卟啉配体17(0.5当量)和用3滴定四吡啶基配体18(0.25当量)分别形成11-卟啉和21-卟啉阵列,含有21种卟啉阵列,其中包含三种不同金属化态的卟啉。内在质子的化学位移以及卟啉配体的吡啶基的邻位和内位质子的化学位移的变化清楚地表明了大的多个卟啉配合物的形成。这些研究表明,通过使用精心设计的构建基块和合适的桥连配体,可以在相对较少的步骤中以大幅度增加尺寸构建卟啉阵列。利用吡啶基配体的结合强度为Ru> Zn> Ni的事实,
表征谱图
-
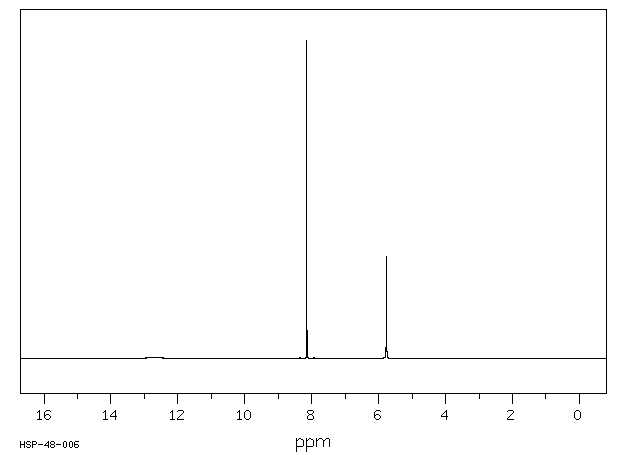
氢谱1HNMR
-
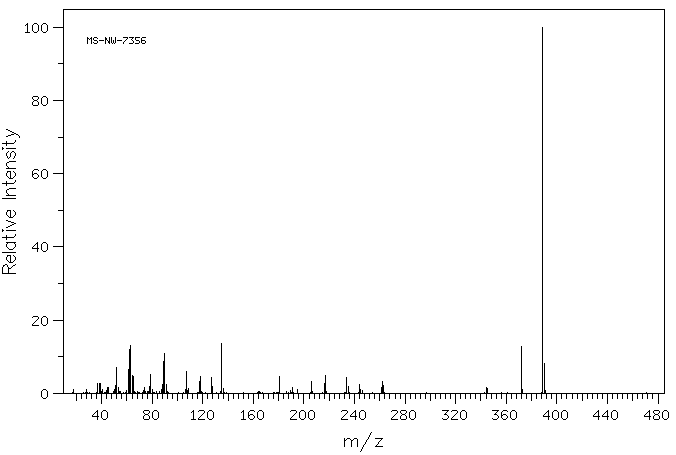
质谱MS
-
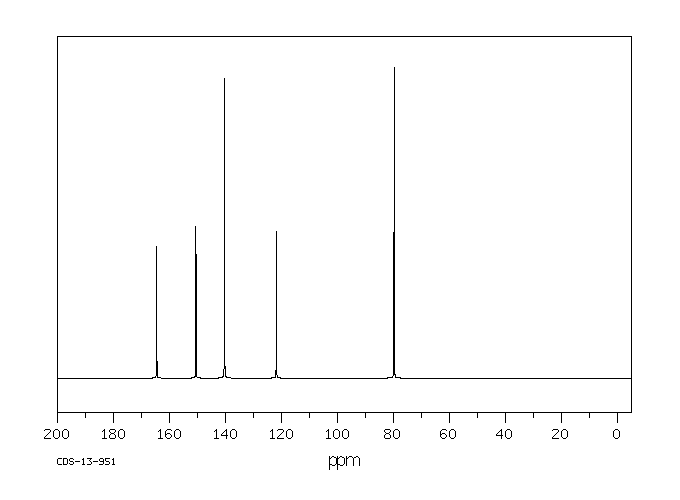
碳谱13CNMR
-
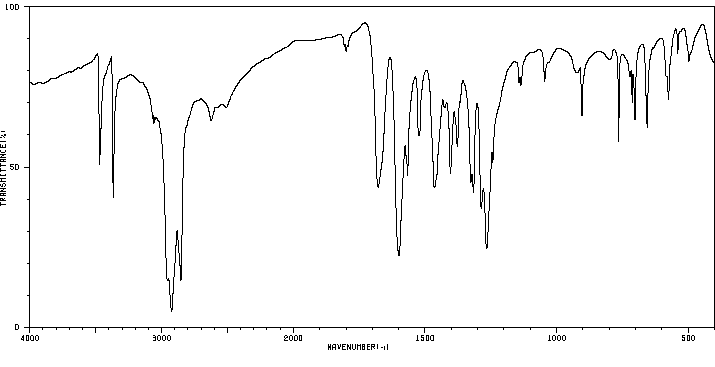
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫