4-氯-3-硝基苯基硫代异氰酸酯 | 127142-66-9
中文名称
4-氯-3-硝基苯基硫代异氰酸酯
中文别名
4-氯-3-硝基苯基硫代异硫氰酸酯;4-氯-3-硝基苯基硫氰酸酯
英文名称
1-chloro-4-isothiocyanato-2-nitrobenzene
英文别名
(4-chloro-3-nitrophenyl)isothiocyanate;4-chloro-3-nitrobenzeneisothiocyanate;4-Chloro-3-nitrophenyl isothiocyanate
CAS
127142-66-9
化学式
C7H3ClN2O2S
mdl
MFCD00041236
分子量
214.632
InChiKey
ZXGZBHIDSJXKLE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64 °C
-
沸点:340.2±27.0 °C(Predicted)
-
密度:1.48±0.1 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。应避免与氧化物、水分等接触。
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:90.3
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险类别码:R23/24/25,R36/37/38
-
危险品运输编号:2811
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
安全说明:S26,S36/37/39,S45
-
储存条件:保持贮藏器密封,并将其存放在阴凉、干燥的地方。确保工作间有良好的通风或排气装置。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-硝基-4-氯苯胺 4-Chloro-3-nitroaniline 635-22-3 C6H5ClN2O2 172.571 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-(4-chloro-3-nitrophenyl)thiourea 373601-07-1 C7H6ClN3O2S 231.663
反应信息
-
作为反应物:描述:参考文献:名称:Continuous-Flow Processing of Gaseous Ammonia Using a Teflon AF-2400 Tube-in-Tube Reactor: Synthesis of Thioureas and In-Line Titrations摘要:A simple tube-in-tube reactor based on the gas-permeable membrane Teflon AF-2400 was used in the continuous flow reaction of gaseous ammonia with isothiocyanates and one isocyanate. A colourimetric in-line titration technique is also reported as a simple method to quantify the amount of ammonia taken up by the solvent in the system.DOI:10.1055/s-0031-1290963
-
作为产物:描述:参考文献:名称:Substituted benzazoles and methods of their use as inhibitors of Raf kinase摘要:提供了新的替代苯唑化合物、组合物和抑制人类或动物主体中Raf激酶活性的方法。这些新化合物组合物可以单独使用,也可以与至少一种额外药物结合,用于治疗由Raf激酶介导的疾病,如癌症。公开号:US20040122237A1
文献信息
-
Antimicrobial and Anti-biofilm Activity of Thiourea Derivatives Bearing 3-amino-1H-1,2,4-triazole Scaffold作者:Joanna Stefanska、Karolina Stepien、Anna Bielenica、Daniel Szulczyk、Barbara Miroslaw、Anna E Koziol、Giuseppina Sanna、Filippo Iuliano、Silvia Madeddu、Michal Jozwiak、Marta StrugaDOI:10.2174/1573406412666151204003146日期:2016.6.233-amino-1H-1,2,4-triazole with the commercial aliphatic and aromatic isothiocyanates. The aliphatic isothiocyanate was used as reagent leading to substitution on NH atom of 3-aminotriazole ring, whereas the triazole amino group was substituted when isothiocyanate group was bonded to the Csp2 hybridized atom, e.g. an aryl or C=O fragment. All compounds were evaluated in vitro for the antimicrobial activity通过使3-氨基-1H-1,2,4-三唑与市售的脂族和芳族异硫氰酸酯反应,制得了21种硫脲衍生物。脂族异硫氰酸酯用作导致3-氨基三唑环的NH原子上取代的试剂,而当异硫氰酸酯基团键合至Csp2杂化原子,例如芳基或C = O片段时,三唑氨基被取代。在体外评估所有化合物的抗微生物活性。衍生物1、2、4、8、9、10和12对革兰氏阳性球菌(金黄色葡萄球菌和表皮葡萄球菌)显示出最高的抑制作用。观察到的MIC值在4–32μg/ mL范围内。还测试了化合物对医院中耐金霉素的金黄色葡萄球菌菌株的体外抗菌活性。观察到的MIC值从4到64μg/ mL不等。产物4和10有效地抑制了耐甲氧西林和表皮葡萄球菌标准菌株的生物膜的形成。与对照相比,发现化合物10的IC50值为2–6μg/ mL更有希望。此外,评估了所有研究的硫脲对MT-4细胞的细胞毒性。化合物18具有明显的细胞毒性(CC50 = 8μM)。
-
[EN] TRIAZOLE COMPOUNDS AS ANTIVIRALS<br/>[FR] COMPOSÉS TRIAZOLES EN TANT QU'ANTIVIRAUX申请人:HOFFMANN LA ROCHE公开号:WO2014006066A1公开(公告)日:2014-01-09The present invention discloses compounds of Formula I: wherein the variables in Formula I are defined as described herein. Also disclosed are pharmaceutical compositions containing such compounds and methods for using the compounds of Formula I in the prevention or treatment of HCV infection.本发明公开了公式I的化合物:其中公式I中的变量定义如本文所述。还公开了包含此类化合物的药物组合物以及使用公式I化合物预防或治疗HCV感染的方法。
-
Thiourea and benzamide compounds, compositions and methods of treating or preventing inflammatory diseases and atherosclerosis申请人:Warner-Lambert Company公开号:US06268387B1公开(公告)日:2001-07-31The present invention provides compounds of formula (I). The present invention also provides methods of treating or preventing inflammation or atherosclerosis, and a pharmaceutical composition that contains a compound of formula (I).本发明提供了化合物的结构式(I)。本发明还提供了治疗或预防炎症或动脉粥样硬化的方法,以及含有结构式(I)化合物的药物组合物。
-
Synthesis and Octopaminergic-agonist Activity of 3-(Substituted Phenyl)imidazolidine-2-thiones and Related Compounds作者:Akinori HIRASHIMA、Kenji SHINKAI、Eiichi KUWANO、Eiji TANIGUCHI、Morifusa ETODOI:10.1271/bbb.62.1179日期:1998.13-(Substituted phenyl)imidazolidine-2-thiones (SPITs) and related compounds were synthesized by cyclizing monoethanolamine hydrogen sulfate with arylisothiocyanates in the presence of sodium hydroxide. The activity for stimulating adenylate cyclase prepared from thoracic nerve cords of the American cockroach, Periplaneta americana L., was examined with these compounds. A SPIT with a 2,6-diethylphenyl通过在氢氧化钠存在下将单乙醇胺硫酸氢盐与芳基异硫氰酸酯环化,可以合成3-(取代的苯基)咪唑烷-2-硫酮(SPIT)和相关化合物。用这些化合物检查了刺激从美国蟑螂美洲大i的胸神经线制备的腺苷酸环化酶的活性。具有2,6-二乙基苯基基团的SPIT(48)是唯一的完全激动剂,其他SPIT衍生物是部分激动剂。更大的酶活化似乎是由短链烷基而不是SPITs芳香环的2,6-位上的卤素取代引起的。在2,6-二取代的SPIT中,从甲基到乙基的链长增加导致酶激活增加。同时,在2中,从乙基到异丙基的链长进一步增加 6-二取代的SPIT导致酶活化降低。能量最小的章鱼胺和48的叠加显示出结构和构象相似,这说明48的Vmax值更高。在有效SPIT的咪唑烷环的C4或C5烷基化后,酶的活化作用明显降低。因此,在SPIT的苯环和N-末端的2-位和6-位的一定程度的蓬松度和疏水性对于活化腺苷酸环化酶是有利的。
-
Substituted Spiro Compounds and Their Use for Producing Pain-Relief Medicaments申请人:Frank Robert公开号:US20080269271A1公开(公告)日:2008-10-30The present invention relates to substituted spiro compounds, to processes for preparing them, to medicaments comprising these compounds and to the use of these compounds for producing medicaments.本发明涉及替代螺环化合物,涉及制备这些化合物的方法,涉及含有这些化合物的药物以及利用这些化合物生产药物的用途。
表征谱图
-
氢谱1HNMR
-
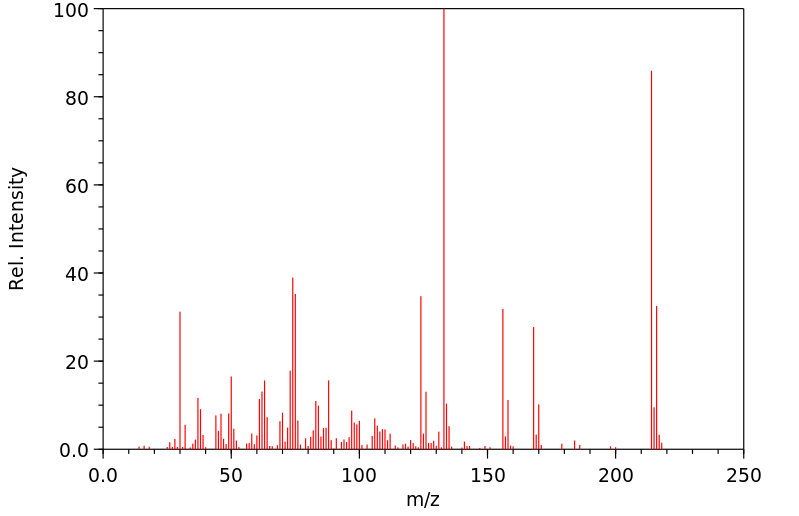
质谱MS
-
碳谱13CNMR
-
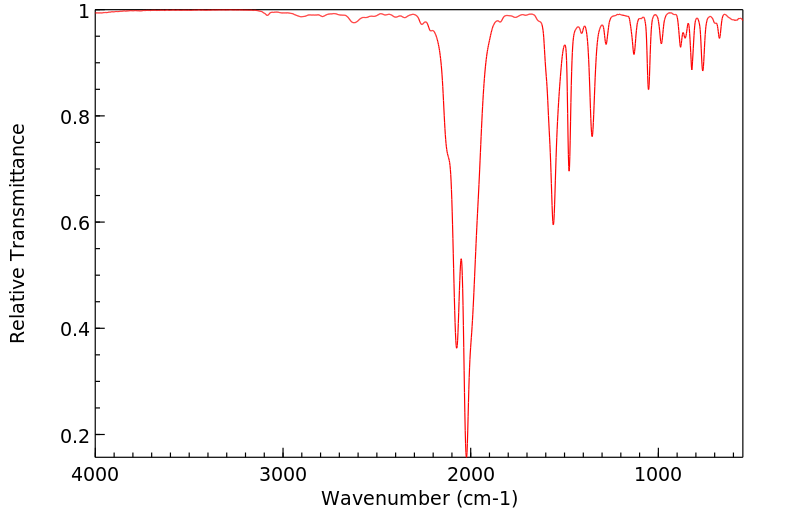
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫