4-溴-2-甲基苯酚 | 2362-12-1
中文名称
4-溴-2-甲基苯酚
中文别名
4-溴邻甲酚;2-甲基-4-溴苯酚;4-溴邻甲基苯酚;4-溴-o-甲酚
英文名称
4-Bromo-2-methylphenol
英文别名
——
CAS
2362-12-1
化学式
C7H7BrO
mdl
MFCD00055435
分子量
187.036
InChiKey
IWJGMJHAIUBWKT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:63-67 °C
-
沸点:63-67°C
-
密度:1.3839 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:IRRITANT, IRRITANT-HARMFUL
-
危险品标志:Xn
-
安全说明:S26,S36/37/39
-
危险类别码:R22
-
海关编码:2908199090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:室温
SDS
4-Bromo-o-cresol Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-Bromo-o-cresol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-Bromo-o-cresol
Percent: ....
CAS Number: 2362-12-1
Synonyms: 4-Bromo-2-methylphenol
Chemical Formula: C7H7BrO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
4-Bromo-o-cresol
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Corect spilled material into an airtight container. Adhered or collected material
containment and cleaning should be promptly disposed of, in accordance with appropriate laws and regulations.
up:
Section 7. HANDLING AND STORAGE
Handling
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Incompartible materials: oxidizing agents
Hazardous Decomposition carbon monoxide, carbon dioxide etc.
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
4-Bromo-o-cresol
Section 11. TOXICOLOGICAL INFORMATION
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. Observe all federal, state and local
regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-Bromo-o-cresol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-Bromo-o-cresol
Percent: ....
CAS Number: 2362-12-1
Synonyms: 4-Bromo-2-methylphenol
Chemical Formula: C7H7BrO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
4-Bromo-o-cresol
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Corect spilled material into an airtight container. Adhered or collected material
containment and cleaning should be promptly disposed of, in accordance with appropriate laws and regulations.
up:
Section 7. HANDLING AND STORAGE
Handling
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Incompartible materials: oxidizing agents
Hazardous Decomposition carbon monoxide, carbon dioxide etc.
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
4-Bromo-o-cresol
Section 11. TOXICOLOGICAL INFORMATION
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. Observe all federal, state and local
regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
4-溴-2-甲基苯酚主要用作有机合成与医药化学中间体。它可用于药物分子和消毒剂的合成以及实验室科学研究。
制备将1 mmol邻甲基苯酚溶于3 mL乙醇中,然后向混合物中加入1.02 mmol重结晶提纯的N-溴代丁二酰亚胺。所得混合物在室温下搅拌1分钟,并用薄层色谱(TLC)监测反应进程,确保原料完全反应后,在减压下除去乙醇溶剂。
接着向反应体系中加入10 mL乙酸乙酯和10 mL蒸馏水,将混合物转移至分液漏斗中。分离有机相(乙酸乙酯),并用2 × 7 mL的乙酸乙酯萃取水层。合并所有乙酸乙酯提取物,并使用无水硫酸镁干燥。最后,通过旋干有机溶剂得到粗产物,再经柱层析分离纯化即可获得目标产物4-溴-2-甲基苯酚。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-4-溴苯甲醚 2-methyl-4-bromoanisole 14804-31-0 C8H9BrO 201.063 5-溴-2-羟基苯甲醇 5-bromo-2-hydroxybenzyl alcohol 2316-64-5 C7H7BrO2 203.035 邻甲酚 ortho-cresol 95-48-7 C7H8O 108.14 3-溴甲基苯 3-bromotoluene 591-17-3 C7H7Br 171.037 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-4-溴苯甲醚 2-methyl-4-bromoanisole 14804-31-0 C8H9BrO 201.063 4-溴-1-乙氧基-2-甲基苯 4-bromo-1-ethoxy-2-methylbenzene 79636-93-4 C9H11BrO 215.09 5-溴-2-羟基-3-甲基苯甲醛 5-bromo-2-hydroxy-3-methylbenzaldehyde 33172-56-4 C8H7BrO2 215.046 —— 4-bromo-2-methyl-1-phenoxybenzene 364354-09-6 C13H11BrO 263.134 —— (4-bromo-2-methyl-phenyl)-propyl ether 132312-53-9 C10H13BrO 229.117 5-溴-2-(二氟甲氧基)甲苯 4-bromo-1-(difluoromethoxy)-2-methylbenzene 888327-32-0 C8H7BrF2O 237.044 —— 5-Brom-2-hydroxy-3-methylbenzylalkohol 77691-33-9 C8H9BrO2 217.062 —— 4-bromo-1-isopropoxy-2-methylbenzene —— C10H13BrO 229.117 —— 2-(4-bromo-2-methylphenoxy)ethan-1-ol 741699-20-7 C9H11BrO2 231.089 —— 1-Allyloxy-4-bromo-2-methylbenzene 309947-09-9 C10H11BrO 227.101 4-溴-2-氯-6-甲基苯酚 4-bromo-2-chloro-6-methyl-phenol 7530-27-0 C7H6BrClO 221.481 邻甲酚 ortho-cresol 95-48-7 C7H8O 108.14 —— 2-amino-4-bromo-6-methylphenol 343269-52-3 C7H8BrNO 202.051 2,4-二溴-6-溴甲基苯酚 2-(bromomethyl)-4,6-dibromophenol 4186-54-3 C7H5Br3O 344.828 —— 4-bromo-1-((2-methoxyethoxy)methoxy)-2-methylbenzene —— C11H15BrO3 275.142 —— 5-Bromo-2-decyloxytoluene 181469-30-7 C17H27BrO 327.305 —— 4-bromo-2-methyl-1-(2-propoxyethoxy)benzene 279262-06-5 C12H17BrO2 273.17 2-苄氧基-5-溴甲苯 1-(benzyloxy)-4-bromo-2-methylbenzene 338454-32-3 C14H13BrO 277.161 2-(4-溴-2-甲基苯氧基)乙酸 4-bromo-2-methylphenoxyacetic acid 6956-82-7 C9H9BrO3 245.073 —— 4-bromo-1-[(2,2-dimethoxyethyl)oxy]-2-methylbenzene —— C11H15BrO3 275.142 —— 3-(4-Bromo-2-methylphenyloxy)propane-1,2-diol 309947-12-4 C10H13BrO3 261.115 —— 4-bromo-2-(bromomethyl)-1-ethoxybenzene 1094438-61-5 C9H10Br2O 293.986 —— 4-Bromo-2-methyl-1-(4-methylbenzyloxy)benzene 854259-10-2 C15H15BrO 291.187 —— 1-(4-bromo-2-methylphenoxy)-2-methylpropan-2-ol 1099665-29-8 C11H15BrO2 259.143 4-溴-2-(叔丁基)-6-甲基苯酚 4-bromo-2-(tert-butyl)-6-methylphenol 25476-59-9 C11H15BrO 243.143 —— (4-Bromo-2-methyl-phenoxy)-acetic acid methyl ester 2867-53-0 C10H11BrO3 259.1 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:多功能球蛋白脱卤过氧化物酶再次出现:EPA污染物氧化中同时的过氧化物酶和过氧化酶机制摘要:研究发现,来自三叶类多毛A蛇Amphitrite ornata的多功能催化血红蛋白脱卤过氧化物酶(DHP)可以催化H 2 O 2依赖性的EPA优先污染物(4- Me - o-甲酚,4-Cl- m-甲酚和五氯酚)的氧化。EPA有毒物质控制法案化合物(ø - ,米- ,p甲酚和4-氯离子ö甲酚)。生化分析(HPLC / LC-MS)表明形成了多种氧化产物,包括相应的邻苯二酚,2-甲基苯醌(2-MeBq)和氧化和/或脱卤程度不同的低聚物。使用4-BR- Ø-甲酚作为代表性底物,用18 O进行标记研究证实掺入邻苯二酚的O原子仅来自H 2 O 2,而掺入2-MeBq的O原子则来自H 2 O,与此一致单个底物分别被过氧合酶和过氧化物酶机制氧化。停止流动的紫外可见光谱研究强烈暗示了化合物I在导致儿茶酚形成的过氧化酶机制中的作用,以及化合物I和ES在产生2-MeBq产物的过氧化物酶机制中的作用。DHP与4-F-邻甲酚(1DOI:10.1016/j.abb.2019.108079
-
作为产物:描述:参考文献:名称:配位聚合物负载单点Cu(II)催化剂的定向结构转变,以控制CH卤化位点的选择性摘要:C–H键功能化的主要困难是在发生反应的地方准确地控制催化剂。在这项工作中,为了获得高效和区域选择性的单中心催化剂,{[Mn(Hidbt)DMF]·H 2 O} n(1)[H 3 idbt = 5的三维(3D)菱形骨架构造了含有配位DMF分子的,5'-(1 H-咪唑-4,5-二基)-双(2 H-四唑)]。对于溶解-再结晶的结构转变过程,有吸引力的结构转变从1到新的晶体形式为{[Mn 3(idbt)2(H 2O)2 ]·3H 2 O} n(2)具有3D窗口状结构,然后可以通过阳离子交换以单晶至单晶的方式将2中的Mn离子与Cu离子交换,从而生成Cu-交换产物{[Mn 2 Cu(idbt)2(H 2 O)2 ]·3H 2 O} n(2a),其窗口状骨架类似于2。此外,2和2a用作非均相催化剂,用于苯酚与N的区域选择性C–H卤化-卤代琥珀酰亚胺(NCS和NBS)来生产定点选择性单卤代产物。发现2a的DOI:10.1021/acs.inorgchem.9b01891
-
作为试剂:描述:参考文献:名称:Fast coupling lemon-yellow phenolic couplers摘要:本发明涉及2,2'-二羟基二苯基硫化物、亚砜和磺酮化合物的替代物,以及它们在光敏重氮组合物中作为偶氮偶联组分的用途。这些新化合物与快速发展的重氮化合物偶联,形成柠檬黄染料,并可与蓝色和紫色偶氮偶联剂结合使用,在高密度区域提供绿黑色的重氮图像,在低密度区域提供中性黑色。公开号:US04642383A1
文献信息
-
Melanocortin-4 receptor binding compounds and methods of use thereof申请人:Millennium Pharmaceuticals, Inc.公开号:US20040082779A1公开(公告)日:2004-04-29Provided are MC4-R binding compounds of the formula XVII: 1 wherein L 2 is a linker group, and P 1 , P 2 , P 3 , P 4 , Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , t, s, and R are as described in the specification. Methods of using the compounds to treat MC4-R associated disorders, such as disorders associated with weight loss, are also provided.
-
1,1,2,2-Tetrahydroperoxy-1,2-Diphenylethane: An efficient and high oxygen content oxidant in various oxidative reactions作者:Kaveh Khosravi、Shirin NaserifarDOI:10.1016/j.tet.2018.09.041日期:2018.11Several oxidative approaches namely thiocyanation of aromatic compounds, epoxidation of alkenes, amidation of aromatic aldehydes, epoxidation of α, β-unsaturated ketones, oxidation of sulfides to sulfoxides and sulfones, bayer-villeger reaction, bromination and iodation of aniline and phenol derivatives oxidative esterification, oxidation of pyridines and oxidation of secondary, allylic and benzyllic
-
[EN] TRIAZOLONE COMPOUNDS AND USES THEREOF<br/>[FR] COMPOSÉS DE TRIAZOLONE ET LEURS UTILISATIONS申请人:INCEPTION 2 INC公开号:WO2013134562A1公开(公告)日:2013-09-12The invention disclosed herein is directed to compounds of Formula (I) and pharmaceutically acceptable salts thereof, which are useful in the treatment of prostate, breast, colon, pancreatic, human chronic lymphocytic leukemia, melanoma and other cancers. The invention also comprises pharmaceutical compositions comprising a therapeutically effective amount of compound of Formula (I), or a pharmaceutically acceptable salt thereof. The invention disclosed herein is also directed to methods of treating prostate, breast, ovarian, liver, kidney, colon, pancreatic, human chronic lymphocytic leukemia, melanoma and other cancers. The invention disclosed herein is further directed to methods of treating prostate, breast, colon, pancreatic, chronic lymphocytic leukemia, melanoma and other cancers comprising administration of a of a therapeutically effective amount of a selective PPARα antagonist. The compounds and pharmaceutical compositions of the invention are also useful in the treatment of viral infections, such as HCV infections and HIV infections. The invention disclosed herein is also directed to a methods of preventing the onset of and/or recurrence of acute and chronic myeloid leukemia, as well as other cancers, comprising administration of a of a therapeutically effective amount of a selective PPARα antagonist.本发明涉及的化合物属于式(I)及其药学上可接受的盐,可用于治疗前列腺、乳腺、结肠、胰腺、人类慢性淋巴细胞白血病、黑色素瘤和其他癌症。该发明还包括含有式(I)化合物的治疗有效量或其药学上可接受的盐的药物组合物。本发明还涉及治疗前列腺、乳腺、卵巢、肝脏、肾脏、结肠、胰腺、人类慢性淋巴细胞白血病、黑色素瘤和其他癌症的方法。本发明还涉及治疗前列腺、乳腺、结肠、胰腺、慢性淋巴细胞白血病、黑色素瘤和其他癌症的方法,包括给予选择性PPARα拮抗剂的治疗有效量。本发明的化合物和药物组合物还可用于治疗病毒感染,如HCV感染和HIV感染。本发明还涉及一种预防急性和慢性骨髓性白血病以及其他癌症发作和/或复发的方法,包括给予选择性PPARα拮抗剂的治疗有效量。
-
Efficient and Regioselective Bromination of Aromatic Compounds with Ethylenebis(<i>N</i>-methylimidazolium) Ditribromide (EBMIDTB)作者:Rahman Hosseinzadeh、Mahmood Tajbakhsh、Maryam Mohadjerani、Zahra LasemiDOI:10.1080/00397910903019975日期:2010.2.26A regioselective and highly efficient method for bromination of phenol and aniline derivatives using ethylenebis(N-methylimidazolium) ditribromide (EBMIDTB) as an efficient reagent in dichloromethane at ambient temperature is reported. The reagent can be recovered and reused several times.
-
Efficient Monofluoroalkylation of Thiophenols or Phenols with α-Bromo-α-Fluoroketones under Mild Conditions作者:Jingjing Wu、Fanhong Wu、Zhi Li、Mougui Fang、Yunli Liu、Yecheng LiuDOI:10.1055/a-1395-4788日期:2021.7nucleophilic substitution reaction between α-bromo-α-fluoroketones and thiophenols or phenols is reported for the synthesis of α-fluoro-β-ketosulfides or α-fluoro-β-ketone ethers in yields ranging from 78–93%. This method exhibits good functional group tolerance and a broad scope of nucleophilic substrates, including natural phenolic compounds.
表征谱图
-
氢谱1HNMR
-
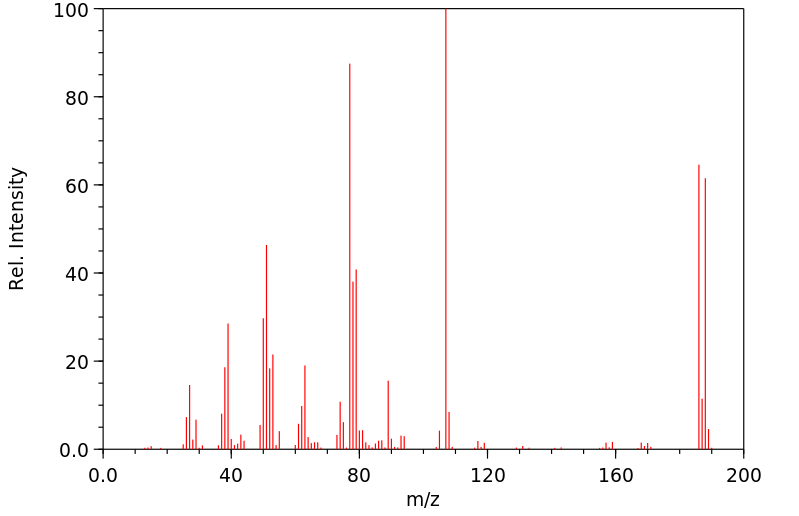
质谱MS
-
碳谱13CNMR
-
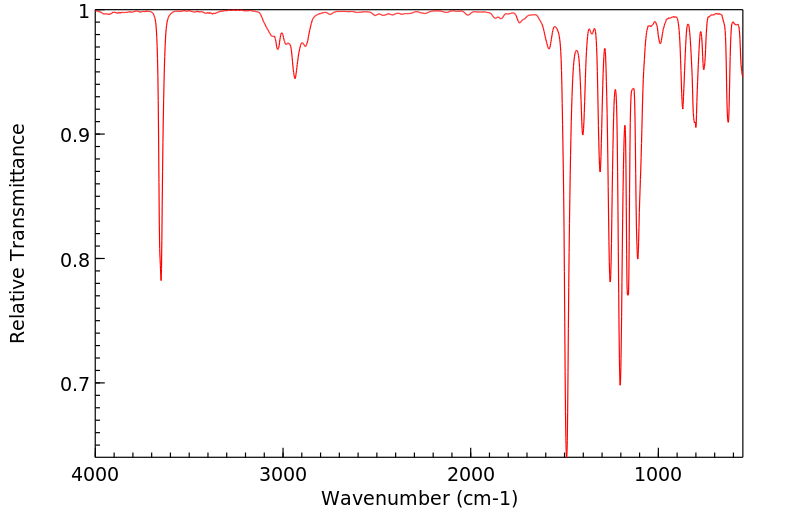
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚