4-甲基-4-苯基-1,3-恶唑烷-2-酮 | 81467-35-8
中文名称
4-甲基-4-苯基-1,3-恶唑烷-2-酮
中文别名
——
英文名称
4-methyl-4-phenyloxazolidin-2-one
英文别名
4-Methyl-4-phenyl-oxazolidin-2-one;4-methyl-4-phenyl-1,3-oxazolidin-2-one
CAS
81467-35-8
化学式
C10H11NO2
mdl
——
分子量
177.203
InChiKey
QXDVUUOJXPDDPQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:79.6-80.0 °C
-
沸点:387.5±22.0 °C(Predicted)
-
密度:1.143±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:2
SDS
反应信息
-
作为产物:描述:2-苯基-1-丙醇 在 silver hexafluoroantimonate 、 sodium azide 、 C19H21CoINO 、 三乙胺 作用下, 以 2,2,2-三氟乙醇 、 丙酮 为溶剂, 反应 39.08h, 生成 4-甲基-4-苯基-1,3-恶唑烷-2-酮参考文献:名称:用于位点选择性分子内 C-H 酰胺化的明确 [Cp*Co(N,O)I]-催化剂摘要:我们提出了一种通过定义明确的 Cp*Co(N,O)I 配合物催化的分子内酰胺化形成 C-N 键的有效方法。该反应在温和的条件下进行,并在不使用庞大的抗衡阴离子或昂贵的氟化溶剂的情况下产生了区域选择性酰胺化产物。Cp*Co(N,O)-I催化剂对分子内苄基、叔和内部C( sp 3 )-H键酰胺化非常有效,但对分子内C( sp 2 )-H键酰胺化无效。DOI:10.1002/adsc.202200385
文献信息
-
NOVEL INHIBITORS申请人:Heiser Ulrich公开号:US20110092501A1公开(公告)日:2011-04-21The invention relates to novel pyrrolidine derivatives of formula (I): wherein R 1 , R 2 and R 3 are as defined herein, as inhibitors of glutaminyl cyclase (QC, EC 2.3.2.5). QC catalyzes the intramolecular cyclization of N-terminal glutamine residues into pyroglutamic acid (5-oxo-prolyl, pGlu*) under liberation of ammonia and the intramolecular cyclization of N-terminal glutamate residues into pyroglutamic acid under liberation of water.
-
[EN] SUBSTITUTED PYRAZOLO[1,5-a]PYRIMIDINE COMPOUNDS AS mTOR INHIBITORS<br/>[FR] COMPOSÉS PYRAZOLO[1,5-A]PYRIMIDINE SUBSTITUÉS UTILISÉS COMME INHIBITEURS DE MTOR申请人:ARRAY BIOPHARMA INC公开号:WO2011029027A1公开(公告)日:2011-03-10Compounds of Formula I: and salts thereof in which R1, R2, R2a, R3, n, X and ring B have the meanings given in the specification, are inhibitors of mTOR and are useful in the treatment of diseases which are sensitive to inhibition of mTOR, such as cancers.公式I的化合物:以及其中R1、R2、R2a、R3、n、X和环B具有说明书中给出的含义的盐,是mTOR的抑制剂,可用于治疗对mTOR抑制敏感的疾病,如癌症。
-
Versatile Cp*Co(III)(LX) Catalyst System for Selective Intramolecular C–H Amidation Reactions作者:Jia Lee、Jeonghyo Lee、Hoimin Jung、Dongwook Kim、Juhyeon Park、Sukbok ChangDOI:10.1021/jacs.0c04448日期:2020.7.15Herein, we report the development of a tailored cobalt catalyst system of Cp*Co(III)(LX) toward intramolecular C-H nitrene insertion of azidoformates to afford cyclic carbamates. The cobalt complexes were easy to prepare and bench-stable, thus offering a convenient reaction protocol. The catalytic reactivity was significantly improved by the electronic tuning of the bidentate LX ligands, and the observed
-
Enzymatic C(sp<sup>3</sup>)-H Amination: P450-Catalyzed Conversion of Carbonazidates into Oxazolidinones作者:Ritesh Singh、Joshua N. Kolev、Philip A. Sutera、Rudi FasanDOI:10.1021/cs5018612日期:2015.3.6stereoselectivity. Mechanistic studies and KIE experiments show that the C–H activation step in these reactions is rate-limiting and proceeds in a stepwise manner, namely, via hydrogen atom abstraction followed by radical recombination. This study expands the reactivity scope of P450-based catalysts in the context of nitrene transfer transformations and provides first-time insights into the mechanism细胞色素 P450 酶可以有效促进碳叠氮化物底物的活化和环化,通过分子内氮宾 C-H 插入反应生成恶唑烷酮。对底物范围的研究表明,虽然苄基/烯丙基 C-H 键最容易被这些生物催化剂胺化,但更强的二级 C-H 键也易于官能化。利用这种“非天然”反应性并在基于指纹的预测的辅助下,可以鉴定细菌 P450 CYP102A1 的改进活性位点变体,以介导两种具有高区域和立体选择性的萜烯天然产物的氨基官能化。机理研究和 KIE 实验表明,这些反应中的 C-H 活化步骤是限速的,并且以逐步的方式进行,即通过氢原子抽提,然后进行自由基重组。这项研究扩大了基于 P450 的催化剂在氮烯转移转化方面的反应范围,并首次深入了解 P450 催化的 C-H 胺化反应的机理。
-
Organotellurium-mediated synthesis of oxazolidin-2-ones from alkenes作者:Nan X. Hu、Yoshio Aso、Tetsuo Otsubo、Fumio OguraDOI:10.1039/c39870001447日期:——Phenyltellurinyl trifluoracetate in combination with ethyl carbamate and boron trifluoride–diethyl ether reacted with alkenes in refluxed 1,2-dichloroethane, regio- and stereo-selectively giving oxazolidin-2-ones in high yields.
表征谱图
-
氢谱1HNMR
-
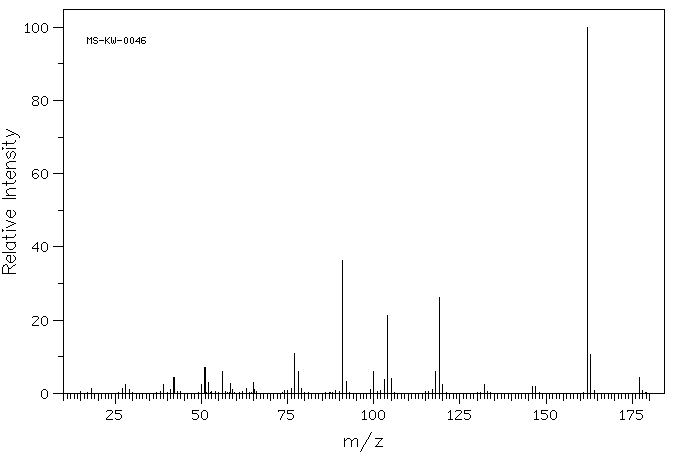
质谱MS
-
碳谱13CNMR
-
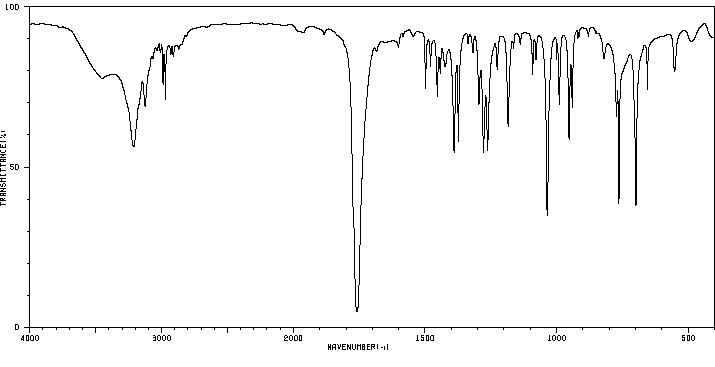
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)