Met-Lys | 45214-88-8
中文名称
——
中文别名
——
英文名称
Met-Lys
英文别名
Methionyl-Lysine;(2S)-6-amino-2-[[(2S)-2-amino-4-methylsulfanylbutanoyl]amino]hexanoic acid
CAS
45214-88-8
化学式
C11H23N3O3S
mdl
——
分子量
277.388
InChiKey
IMTUWVJPCQPJEE-IUCAKERBSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:545.0±50.0 °C(Predicted)
-
密度:1.191±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-3.4
-
重原子数:18
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:0.82
-
拓扑面积:144
-
氢给体数:4
-
氢受体数:6
SDS
反应信息
-
作为反应物:参考文献:名称:通过HOBr / H 2 Se共轭体系对肽和NC1六聚体中亚硫胺键的循环调节摘要:在胶原蛋白IV支架中发现的亚硫胺键(-S═N-)通过形成亚硫胺交联键来显着稳定体系结构。然而,精确控制生物体中亚硫亚胺键的形成和分解过程以实现更好的生命功能仍然是一个挑战。因此,我们建立了一种通过硒化氢(H 2 Se)和次溴酸(HOBr)调节硫亚胺键断裂和形成的新方法,该方法可以在模拟的生理条件下轻松控制。这种新颖的策略提供了一种循环调节系统,可调节肽和NC1六聚体中的亚硫胺键,这可为进一步研究胶原IV的生理功能提供一个实质性的系统。DOI:10.1021/acs.analchem.8b02228
-
作为产物:描述:7-(N-formylmethionyl-L-lysyl)amino-4-methylcoumarin 在 Escherichia coli peptide deformylase 、 HEPES buffer 、 1,4-二巯基-2,3-丁二醇 作用下, 生成 7-氨基-4-甲基香豆素 、 Met-Lys参考文献:名称:一种新的分光光度测定法揭示了环拟肽对肽去甲酰基酶的缓慢结合抑制作用。摘要:通过将PDF反应与二肽基肽酶I(DPPI)偶联并使用N-甲酰基-Met-Lys-AMC作为底物,开发了一种新的分光光度法/荧光法测定肽去甲酰基酶(PDF)。通过PDF去除N-末端甲酰基使二肽成为DPPI的有效底物,其随后去除二肽基单元以释放7-氨基-4-甲基香豆素作为生色团/荧光团。PDF反应可在UV-Vis分光光度计或荧光计上以连续方式方便地进行监控。通过测定PDF的催化活性和PDF抑制剂的抑制常数证明了该测定法的实用性。这些研究揭示了以前报道的大环PDF抑制剂的慢结合行为。DOI:10.1016/j.bioorg.2004.01.001
文献信息
-
Characterization by mass spectrometry and IRMPD spectroscopy of the sulfoxide group in oxidized methionine and related compounds作者:Marta Ignasiak、Debora Scuderi、Pedro de Oliveira、Tomasz Pedzinski、Yamina Rayah、Chantal Houée LevinDOI:10.1016/j.cplett.2010.12.012日期:2011.1Methionine protein residues are prone to oxidation. To unravel the controversy about the mechanism of its one-electron oxidation, we characterised the main biological product, methionine sulfoxide, using mass spectrometry and IR multiple photon dissociation spectroscopy.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
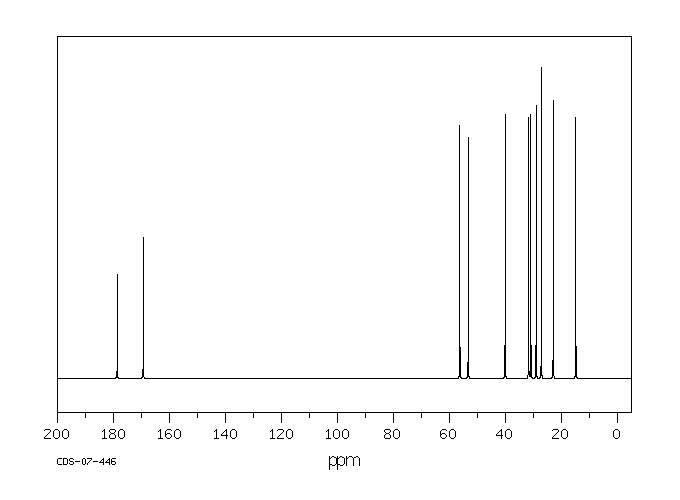
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸