4-联苯基-6-苯甲酸苯酯 | 17064-47-0
中文名称
4-联苯基-6-苯甲酸苯酯
中文别名
2-(4-联苯基)-6-苯基苯丙噁唑;2-(4-联苯)-6-苯基苯并噁唑
英文名称
2-(4-biphenylyl)-6-phenylbenzoxazole
英文别名
2-(biphenyl-4-yl)-6-phenylbenzoxazole;2-(4-biphenyl-3-ido)-5-phenylbenzoxazole;2-biphenyl-4-yl-6-phenyl-benzooxazole;2-(4-biphenylyl)-6-phenyl-1,3-benzoxazole;6-phenyl-2-(4-phenylphenyl)-1,3-benzoxazole
CAS
17064-47-0
化学式
C25H17NO
mdl
MFCD00005768
分子量
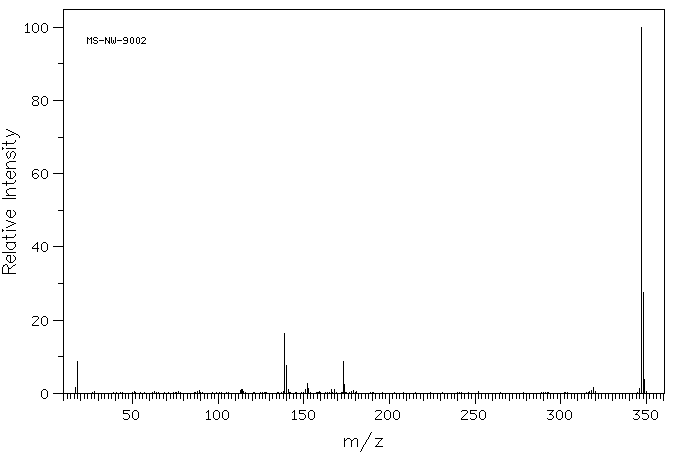
347.416
InChiKey
WISWLZYHZLVSMO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:198-199 °C(lit.)
-
沸点:481.96°C (rough estimate)
-
密度:1.1510 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):6.7
-
重原子数:27
-
可旋转键数:3
-
环数:5.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:26
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
WGK Germany:3
-
海关编码:2934999090
制备方法与用途
用途:适宜用作激光染料。
反应信息
-
作为反应物:描述:4-联苯基-6-苯甲酸苯酯 、 氯化铑(三水) 在 ethylenediamine 作用下, 以 乙二醇甲醚 为溶剂, 以56%的产率得到bis[2-(biphenyl-4-yl-3-ido)-6-phenylbenzoxazole]ethylenediaminerhodium(III) chloride参考文献:名称:Cyclometalated Pd(ii) and Rh(iii) complexes with ethylenediamine based on 2-(4-biphenyl)-5-phenylbenzoxazole luminophore摘要:DOI:10.1134/s1070363211110259
文献信息
-
Chemical address tags申请人:Rosania R. Gustavo公开号:US20070111251A1公开(公告)日:2007-05-17The present invention provides methods and compositions related to the fields of chemoinformatics, chemogenomics, drug discovery and development, and drug targeting. In particular, the present invention provides subcellular localization signals (e.g., chemical address tags) that influence (e.g., direct) subcellular and organelle level localization of associated compounds (e.g., drugs and small molecule therapeutics, radioactive species, dyes and imagining agents, proapoptotic agents, antibiotics, etc) in target cells and tissues. The compositions of the present invention modulate the pharmacological profiles of associated compounds by influencing the compound's accumulation, or exclusion, from subcellular loci such as mitochondria, endoplasmic reticulum, cytoplasm, vesicles, granules, nuclei and nucleoli and other subcellular organelles and compartments. The present invention also provides methods for identifying chemical address tags, predicting their targeting characteristics, and for rational designing chemical libraries comprising chemical address tags.
-
Organic electroluminescent devices having improved power conversion efficiencies申请人:EASTMAN KODAK COMPANY (a New Jersey corporation)公开号:EP0120673A2公开(公告)日:1984-10-03Electroluminescent devices are disclosed comprising a hole-injecting zone and an adjacent organic luminescent zone, having a combined thickness no greater than about 1 micron, which have a power conversion efficiency of at least 9 x 10-5 w/w. Preferred electron-transporting compounds for the luminescentzone provide a maximum electroluminescent quantum efficiency of at least 5 ×10-4 photons/electron when tested as described.已公开的电致发光器件包括一个空穴注入区和一个相邻的有机发光区,其总厚度不超过约 1 微米,功率转换效率至少为 9 x 10-5 w/w。用于发光区的优选电子传递化合物在进行所述测试时,可提供至少 5 ×10-4 光子/电子的最大电致发光量子效率。
-
Polylactide compounds as sensitivity enhancers for radiation sensitive mixtures申请人:OCG MICROELECTRONIC MATERIALS, INC.公开号:EP0561625A1公开(公告)日:1993-09-22A radiation-sensitive composition comprises an admixture in a solvent of: at least one alkali-soluble binder resin, at least one photoactive compound and an effective sensitivity enhancing amount of at least one polylactide compound; the amount of said binder resin being about 60 to 95% by weight, the amount of said photoactive component being about 5% to about 40% by weight, based on the total solids content of said radiation-sensitive compostion.
-
High ortho-ortho bonded novolak binder resins and their use in radiation-sensitive compositions申请人:OCG MICROELECTRONIC MATERIALS, INC.公开号:EP0623633A2公开(公告)日:1994-11-09A phenolic novolak composition prepared by a process characterized by the steps of: (1) reacting a first phenolic monomer comprising a major portion of at least one trifunctional phenolic monomer with a first aldehyde source in the absence of a catalyst at a reaction temperature from about 100°C to about 200°C and at a reaction pressure of about 2 to about 15 atmospheres to form a phenolic oligomer having a weight average molecular weight from about 500 to about 2,000, having ortho-ortho bonding of about 55% to about 75% of the methylene bonds between the phenolic moieties; and having a time to clear of less than 125 seconds per micron; wherein the mole ratio of said first aldehyde source to said first phenolic monomer is from about 0.3:1.0 to about 0.55:1.0; and (2) then reacting said oligomer with an optional second phenolic source and a second aldehyde source at a temperature from about 80°C to about 150°C to form a phenolic novolak having a weight average molecular weight of 3,000 to 40,000, having ortho-ortho bonding of between 50% and 70% of the methylene bonds between the phenolic moieties, and having a time to clear of at least 20 seconds per micron; wherein the mole ratio of said second aldehyde source to said total phenolic moieties is less than about 0.8:1.0.一种酚醛树脂组合物,其制备过程包括以下步骤 (1) 在没有催化剂的情况下,在反应温度约为 100°C 至约 200°C,反应压力约为 2 至约 15 个大气压的条件下,使包含至少一种三官能酚类单体的主要部分的第一酚类单体与第一醛源反应,以形成酚类低聚物,该酚类低聚物的重量平均分子量约为 500 至约 2,000,酚基之间的亚甲基键的正交键合率约为 55% 至约 75%;其中所述第一醛源与所述第一酚单体的摩尔比为约 0.3:1.0至约0.55:1.0;以及 (2) 然后将所述低聚物与任选的第二酚源和第二醛源在约80℃至约150℃的温度下反应,以形成酚醛新酯,其重量平均分子量为3,000至40,000,酚基之间50%至70%的亚甲基键为正交键,且每微米的透明时间至少为20秒;其中所述第二醛源与所述酚基总量的摩尔比小于约0.8:1.0。
-
Selected trinuclear novolak oligomers and their use in photo-active compounds and radiation sensitive mixtures申请人:OCG MICROELECTRONIC MATERIALS, INC.公开号:EP0685766A1公开(公告)日:1995-12-06A trinuclear novolak oligomer characterized by the formula (I): wherein each X is selected from the group consisting of hydrogen, hydroxyl group and halide group and Y is selected from the group consisting of a lower alkyl group having 1-4 carbon atoms and a halogen atom.一种三核新酚醛低聚物,其特征为式 (I): 其中每个 X 选自氢、羟基和卤化物基团组成的组,Y 选自具有 1-4 个碳原子的低级烷基和卤素原子组成的组。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
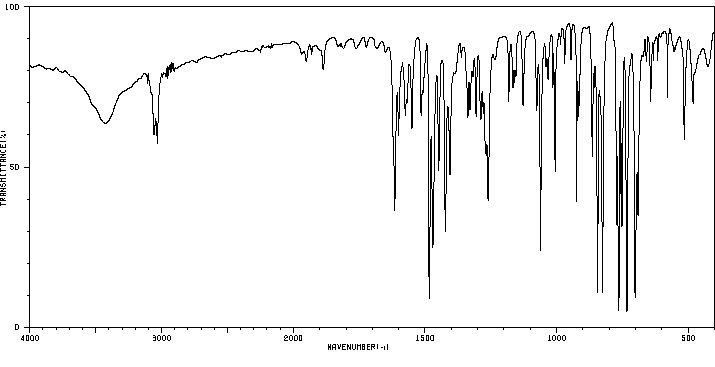
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫