4-脲基-苯甲酸 | 6306-25-8
中文名称
4-脲基-苯甲酸
中文别名
——
英文名称
4-ureidobenzoic acid
英文别名
p-ureidobenzoic acid;4-ureido-benzoic acid;4-Ureido-benzoesaeure;(4-Carboxy-phenyl)-harnstoff;4-carbamoylaminobenzoic acid;4-(1-Ureido)benzoic acid;4-[(Aminocarbonyl)amino]benzoic acid;4-(carbamoylamino)benzoic acid
CAS
6306-25-8
化学式
C8H8N2O3
mdl
MFCD00025432
分子量
180.163
InChiKey
HOVRQUHNZHBKKJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:92.4
-
氢给体数:3
-
氢受体数:3
安全信息
-
海关编码:2924299090
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 4-ureidobenzoate 30932-68-4 C9H10N2O3 194.19 对氨基苯甲酸 4-amino-benzoic acid 150-13-0 C7H7NO2 137.138 4-氨基苯甲酸甲酯 4-methoxycarbonyl aniline 619-45-4 C8H9NO2 151.165 4-异氰酰基苯甲酸甲酯 methyl 4-isocyanatobenzoate 23138-53-6 C9H7NO3 177.159 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(肼羰基氨基)苯甲酸 4-(4-carboxy-phenyl)semicarbazide 73469-95-1 C8H9N3O3 195.178 —— N-(4-carbethoxyphenyl)urea 13289-38-8 C10H12N2O3 208.217
反应信息
-
作为反应物:参考文献:名称:Skin disorders and thyroid diseases摘要:Thyroid disorders have a high prevalence in medical practice; they are associated with a wide range of diseases with which they may or may not share etiological factors. One of the organs which best show this wide range of clinical signs is the skin. This review is an attempt to approach most of the dermopathies reflecting several degrees of harmfulness, coming directly or indirectly from thyroid abnormalities, as well as to update current knowledge on the relationship between the thyroid and skin. We have proposed a primary classification of skin disorders, regarding thyroid involvement, into two main groups: 1) dermopathies associated with thyroid abnormalities, mainly with autoimmune thyroid diseases, like melasma, vitiligo, Sjogren's syndrome, alopecia, idiopathic hirsutism, pre-menstrual acne, bullous diseases, connective tissue diseases, hamartoma syndrome, atopy, leprosy and DiGeorge anomaly; and 2) dermopathies depending on the nature of the thyroid disorder, in which the evolution and outcome of the skin disorder depend on the thyroidal treatment in most cases, such as trophism and skin blood flow, myxedema, alopecia, onychodystrophy, hypo- and hyperhidrosis, xanthomas, intraepidermal bullae, carotenodermia, pruritus, flushing, pyodermitis, palmoplantar keratoderma, ecchymosis, etc. In some other cases, the skin disease which developed as a consequence of the thyroid abnormality can remain unaltered despite functional treatment of the thyroid problem, such as pretibial myxedema, thyroid acropachy and some cutaneous manifestations of multiple endocrine neoplasia types 2A and 2B. (J. Endocrinol. Invest. 24: 628-638, 2001) (C) 2001, Editrice Kurtis.DOI:10.1007/bf03343905
-
作为产物:描述:参考文献:名称:[EN] ANTI-PCSK9 COMPOUNDS AND METHODS FOR THE TREATMENT AND/OR PREVENTION OF CARDIOVASCULAR DISEASES
[FR] COMPOSÉS ANTI-PCSK9 ET MÉTHODES DE TRAITEMENT ET/OU DE PRÉVENTION DE MALADIES CARDIO-VASCULAIRES摘要:揭示了调节前蛋白酶转化亚硫酸酯酶基因型9(PCSK9)生理作用的化合物,以及利用这些调节剂降低低密度脂蛋白胆固醇水平和/或用于治疗和/或预防心血管疾病(CVD),包括治疗高胆固醇血症的方法。公开号:WO2014150395A1
文献信息
-
Protecting Group Free Synthesis of Carboxyl-substituted Dihydropyrimidines Through Biginelli Reaction作者:Eugeniy N. Ostapchuk、Andrey S. Plaskon、Oleksandr O. Grygorenko、Andrey A. Tolmachev、Sergey V. RyabukhinDOI:10.1002/jhet.1568日期:2013.11acetoacetic acid derivatives, and various carboxyl‐containing ureas was explored. It was found that the steric load of the urea substituents influenced strongly the reaction outcome; in particular, the method was efficient only in the case of unbranched mono‐substituted ureas bearing either aliphatic or aromatic groups. The method allows performing a one‐pot, protecting group free synthesis of dihydropyrimidines
-
[EN] NOVEL CYSTOBACTAMIDE DERIVATIVES<br/>[FR] NOUVEAUX DÉRIVÉS DE CYSTOBACTAMIDES申请人:HELMHOLTZ ZENTRUM INFEKTIONSFORSCHUNG GMBH公开号:WO2019038405A1公开(公告)日:2019-02-28The present invention relates to novel derivatives of cystobactamides of formula (lb) and the use thereof for the treatment or prophylaxis of bacterial infections.本发明涉及公式(lb)的半胱菌肽的新颖衍生物及其用于治疗或预防细菌感染的用途。
-
Penicillins申请人:Bayer Aktiengesellschaft公开号:US03931153A1公开(公告)日:1976-01-06Penicillins of the formula: ##SPC1## And their pharmaceutically acceptable nontoxic salts wherein R.sub.1 is hydrogen, halogen, lower alkyl, hydroxy, nitro or A--NH; A is ##EQU1## wherein R.sub.3 is hydrogen; lower alkyl; halo-(lower alkyl); cycloalkyl of 3 to 11 carbon atoms, unsubstituted or substituted by hydroxy or alkyl of 1 or 2 carbon atoms; cycloalkenyl of 3 to 11 carbon atoms; bicycloalkyl of up to 8 carbon atoms; bicycloalkenyl of up to 8 carbon atoms; aryl of 6 to 10 carbon atoms, unsubstituted or substituted by 1 to 3 substituents selected from the group consisting of alkyl of 1 to 4 carbon atoms, alkoxy of 1 to 4 carbon atoms, halogen, trifluoromethyl, nitro, amino, alkylsulphonyl of 1 to 4 carbon atoms, and methylenedioxy; azidoaryl of 6 to 10 carbon atoms, azido-(lower alkyl); amino; or thienyl; R.sub.4 is lower alkylamino or arylamino of 6 to 10 carbon atoms; B is a direct bond; CH.sub.2 ; S--CH.sub.2 ; CH=CH; or CO--NH--CH.sub.2 ; E is phenyl or phenyl or thenyl, substituted by hydroxy, azido, lower alkyl, lower alkoxy, lower alkylthio or chlorine; and C* is an asymmetric carbon atom, Are useful for their antibacterial activity against both Gram-positive and Gram-negative bacteria.公式为:##SPC1## 的青霉素及其药学上可接受的无毒盐,其中 R.sub.1 是氢、卤素、较低的烷基、羟基、硝基或 A--NH;A 是 ##EQU1## 其中 R.sub.3 是氢;较低的烷基;卤代-(较低的烷基);3到11个碳原子的环烷基,未取代或取代为羟基或1或2个碳原子的烷基;3到11个碳原子的环烯基;多环烷基,最多含8个碳原子;多环烯基,最多含8个碳原子;6到10个碳原子的芳基,未取代或取代为1到3个取代基,所述取代基选自1到4个碳原子的烷基、1到4个碳原子的烷氧基、卤素、三氟甲基、硝基、氨基、1到4个碳原子的烷基磺酰基和亚甲二氧基;6到10个碳原子的偶氮芳基,偶氮-(较低的烷基);氨基;或噻吩基;R.sub.4 是6到10个碳原子的较低的烷基氨基或芳基氨基;B 是直接键;CH.sub.2;S--CH.sub.2;CH=CH;或 CO--NH--CH.sub.2;E 是苯基或苯基或噻吩基,取代为羟基、偶氮基、较低的烷基、较低的烷氧基、较低的烷基硫基或氯;C* 是一个不对称碳原子,对其抗菌活性对抗革兰氏阳性和阴性细菌均有用。
-
Substituted benzene derivatives useful as neuraminidase inhibitors申请人:BioCryst Pharmaceuticals, Inc.公开号:US05602277A1公开(公告)日:1997-02-11A compound of the Formula (I): ##STR1## or pharmaceutically-suitable salts or prodrug forms thereof, wherein: n is 0-1; m is 0; p is 0-1; R.sup.1 is --CO.sub.2 H; R.sup.2 is selected from the group consisting of H, --OH, and --NH.sub.2 ; R.sup.3 is H; R.sup.4 is --C(O)NHR.sup.8 ; R.sup.5 is --NHC(R.sup.6)NH.sub.2 R.sup.6 is selected from the group consisting of .dbd.NH, .dbd.NOH, .dbd.NCN, .dbd.O, and .dbd.S; and R.sup.8 is selected from the group consisting of C.sub.1 -C.sub.4 linear or branched alkyl substituted with 0-3 halogens on each carbon.化合物的化学式(I):##STR1##或其药用盐或前药形式,其中:n为0-1;m为0;p为0-1;R.sup.1为--CO.sub.2 H;R.sup.2选自H、--OH和--NH.sub.2的组;R.sup.3为H;R.sup.4为--C(O)NHR.sup.8;R.sup.5为--NHC(R.sup.6)NH.sub.2 R.sup.6选自.dbd.NH、.dbd.NOH、.dbd.NCN、.dbd.O和.dbd.S的组;R.sup.8选自每个碳上取代有0-3卤素的C.sub.1-C.sub.4直链或支链烷基的组。
-
A practically simple, catalyst free and scalable synthesis of <i>N</i>-substituted ureas in water作者:Lata Tiwari、Varun Kumar、Bhuvesh Kumar、Dinesh MahajanDOI:10.1039/c8ra03761b日期:——A practically simple, mild and efficient method is developed for the synthesis of N-substituted ureas by nucleophilic addition of amines to potassium isocyanate in water without organic co-solvent. Using this methodology, a variety of N-substituted ureas (mono-, di- and cyclic-) were synthesized in good to excellent yields with high chemical purity by applying simple filtration or routine extraction
表征谱图
-
氢谱1HNMR
-
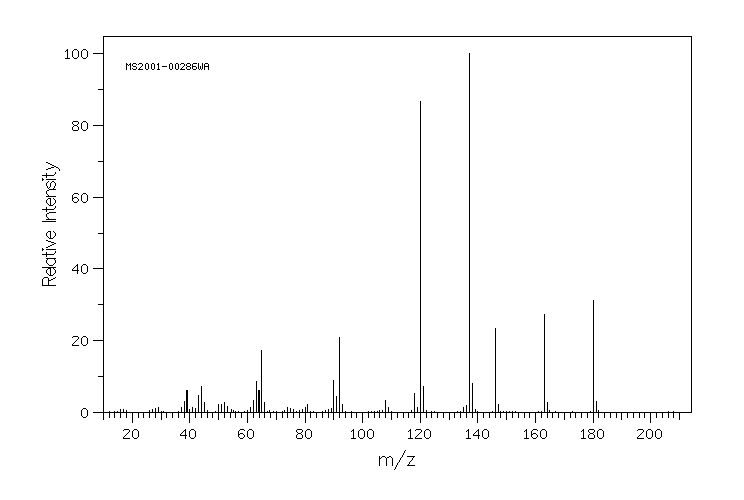
质谱MS
-
碳谱13CNMR
-
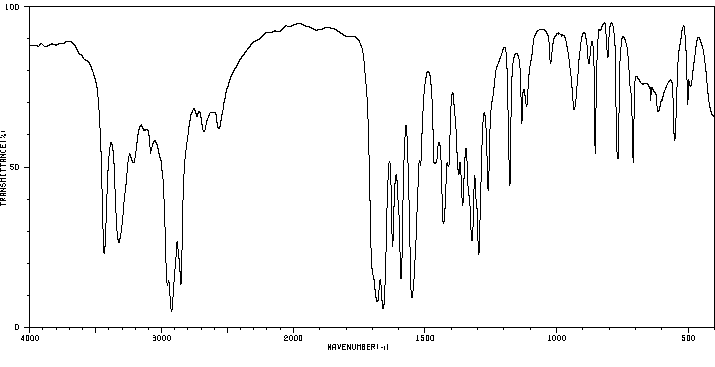
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫