4-methanesulfonylmorpholine | 1697-34-3
中文名称
——
中文别名
——
英文名称
4-methanesulfonylmorpholine
英文别名
4-(methylsulfonyl)morpholine;Morpholine, 4-(methylsulfonyl)-;4-methylsulfonylmorpholine
CAS
1697-34-3
化学式
C5H11NO3S
mdl
MFCD00091634
分子量
165.213
InChiKey
GVZKQMUSVBGSIZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:90-91 °C
-
沸点:278.5±50.0 °C(Predicted)
-
密度:1.31±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2934999090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:取代乙炔磺酰胺的简单通用合成摘要:通过两步操作制备了各种取代的2-芳基乙炔磺酰胺:1.通过适当的甲磺酰胺碳负离子与取代的苯甲酸酯反应制备2-氧代-2-芳基乙磺酰胺,2.通过2-脱水生成的酮氯-N-甲基吡啶碘化物/ NEt 3。DOI:10.1016/s0040-4039(00)97493-8
-
作为产物:描述:参考文献:名称:Preparation of sulfonamides from N-silylamines摘要:Sulfonamides have been prepared in high yields by the reactions of N-silylamines with sulfonyl chlorides and fluorides. In a competition experiment, the sulfonyl chlorides were found to be far more reactive than sulfonyl fluorides. The chemistry may be used to prepare aliphatic, aromatic, tertiary, secondary, and primary sulfonamides. It may also be done in the absence of solvent and the byproduct trimethylsilyl chloride recovered in good yield. Primary sulfonamides were synthesized from the sulfonyl chloride with aminotriphenyl silane (Ph3SiNH2), a conversion demonstrated with the synthesis of the carbonic anhydrase inhibitor, acetazolamide. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2013.08.034
-
作为试剂:描述:methyl 9-isopropyl-1,6-dimethyl-4-[(morpholinosulfonyl)acetyl]heptalene-5-carboxylate 在 正丁基锂 、 4-methanesulfonylmorpholine 作用下, 以 四氢呋喃 为溶剂, 反应 2.0h, 以68%的产率得到9-isopropyl-7,12-dimethyl-3-(morpholinosulfonyl)benzo[a]heptalene-2,4-diol参考文献:名称:一种将七庚烯-4,5-二羧酸酯转化为苯并[ a ]庚烷的“一锅”加油方法†摘要:已经发现当用4mol当量处理时,二甲基庚烯-4,5-二羧酸酯。锂化的N,N-二烷基氨基甲基砜或甲基苯基砜,然后4摩尔当量。在-78至20°的温度范围内,BuLi在THF中的生成会导致生成3-[[(N,N-二烷基氨基)磺酰基]-或3-(苯基磺酰基)苯并[ a ]庚二烯-2, 4-二醇的。(请参阅方案4以及表2和表3)。伴随产物是2,4-双{[((N,N-二烷基氨基)磺酰基]甲基}-或2,4-双[(苯磺酰基)甲基] -4,10a-二氢-3H-庚烯[ 1,10- bc呋喃-3-羧酸酯为非对映异构体的混合物。cf. 方案4和(表2和3)是锂化的甲基砜在庚烯-4,5-二羧酸酯的C(3)处的迈克尔加成反应,然后是甲氧基羰基的(磺酰基)甲基化的结果。 C(5)和环化。(请参阅方案5)。假定苯并[ a ]庚烯的形成是由于庚烯的4,5-二羧酸的庚烷的两个甲氧基羰基的(磺酰基)甲基化。(请参阅方案6和8)。所DOI:10.1002/hlca.19970800821
文献信息
-
β-Cyclodextrin-Catalyzed Monosulfonylation of Amines and Amino Acids in Water作者:R. Sridhar、B. Srinivas、V. Pavan Kumar、M. Narender、K. Rama RaoDOI:10.1002/adsc.200600652日期:2007.8.6A mild and efficient procedure has been developed for the first time under biomimetic conditions for the monosulfonylation of various amines and amino acids catalyzed by β-cyclodextrin in water at room temperature to afford the corresponding sulfonamides in high yields.
-
PTAB mediated open air synthesis of sulfonamides, thiosulfonates and symmetrical disulfanes作者:Debayan Sarkar、Manoj Kumar Ghosh、Nilendri RoutDOI:10.1016/j.tetlet.2018.05.017日期:2018.6A facile methodology has been described which has successfully simplified the generation of sulfonamides, thiosulfonates and symmetric disulfanes. This “trio” of reactions occur in an open air metal free atmosphere and has also been scaled up to grams making it suitable for commercialization. The reactions also have been successfully carried out with asymmetric variants, thus contributing to the chiral
-
Base-free monosulfonylation of amines using tosyl or mesyl chloride in water作者:Ahmed Kamal、J. Surendranadha Reddy、E. Vijaya Bharathi、D. DastagiriDOI:10.1016/j.tetlet.2007.11.044日期:2008.1A mild and efficient procedure has been developed for the monosulfonylation of various amines using mesyl or tosyl chlorides in water at room temperature to afford the corresponding sulfonamides in high yields.
-
Copper-catalyzed electrophilic amination of sodium sulfinates at room temperature作者:Haibo Zhu、Yajing Shen、Qinyue Deng、Tao TuDOI:10.1039/c5cc06069a日期:——By using O-benzoyl hydroxylamines as amine source, the first convenient copper-catalyzed electrophilic amination of sodium sulfinates has been realized. Even with 2 mol% catalyst loading, the protocol provided an efficient...
-
Leishmanicidal potential of N-substituted morpholine derivatives: Synthesis and structure–activity relationships作者:Khalid Mohammed Khan、Muhammad Zarrar Khan、Muhammad Taha、Ghulam Murtaza Maharvi、Zafar Saeed Saify、Shahnaz Parveen、Muhammad Iqbal ChoudharyDOI:10.1080/14786410802090359日期:2009.3.20A series of N-substituted morpholines 2–20 was synthesised by reacting various acid chlorides and alkyl halides with morpholine (1). All of the synthesised compounds 2–20 were screened for their leishmanicidal effects using amphotericin B (IC50 = 0.24 µg L−1) and pentamidine (IC50 = 2.56 µg mL−1) as standards and a structure–activity relationship (SAR) study was established. The compounds 2 (IC50 =一系列的N-取代的吗啉的2 - 20用各种酰氯和烷基卤化物与吗啉(反应合成1)。所有合成的化合物2 - 20进行了筛选用两性霉素B(IC它们leishmanicidal效果50 = 0.24微克大号-1)和喷他脒(IC 50 = 2.56微克毫升-1)作为标准和结构-活性关系(SAR)研究成立。化合物2(IC 50 = 48 µg mL -1),3(IC 50 = 30.0 µg mL -1),10(IC 50 = 41.0μgmL -1),15(IC 50 = 33.0μgmL -1),16(IC 50 = 35.0μgmL -1)和20(IC 50 = 47.0μgmL -1)表现出较弱的杀菌作用。
表征谱图
-
氢谱1HNMR
-
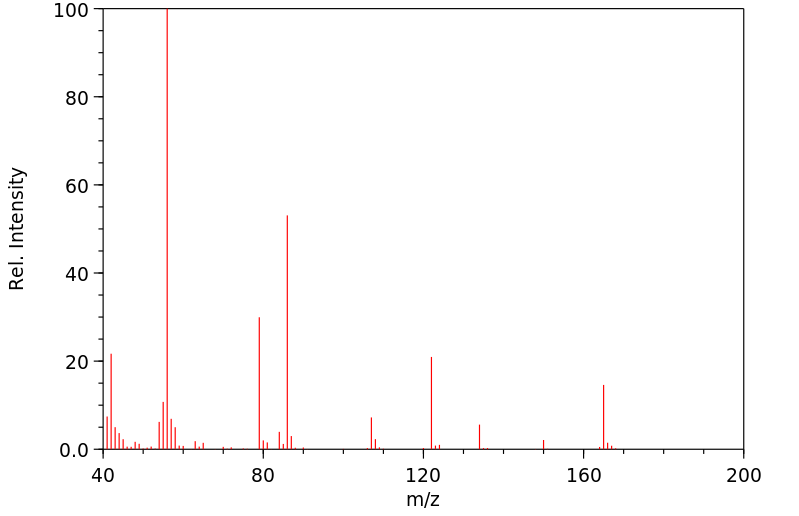
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4-甲基环戊-1-烯-1-基)(吗啉-4-基)甲酮
(2-肟基-氰基乙酸乙酯)-N,N-二甲基-吗啉基脲六氟磷酸酯
鲸蜡基乙基吗啉氮鎓乙基硫酸盐
马啉乙磺酸钾
预分散OTOS-80
顺式4-(氮杂环丁烷-3-基)-2,2-二甲基吗啉
顺式-N-亚硝基-2,6-二甲基吗啉
顺式-3,5-二甲基吗啉
顺-2,6-二甲基-4-(4-硝基苯基)吗啉
非屈酯
雷奈佐利二聚体
阿瑞杂质9
阿瑞杂质12
阿瑞吡坦磷的二卞酯
阿瑞吡坦杂质
阿瑞吡坦杂质
阿瑞吡坦EP杂质C
阿瑞吡坦
阿瑞吡坦
阿瑞匹坦非对映异构体2R3R1R
阿瑞匹坦杂质A异构体
阿瑞匹坦杂质54
阿瑞匹坦-M3代谢物
钾[2 - (吗啉- 4 -基)乙氧基]甲基三氟硼酸
酮康唑杂质
邻苯二甲酸单吗啉
调节安
试剂2-(4-Morpholino)ethyl2-bromoisobutyrate
茂硫磷
苯甲腈,2-(4-吗啉基)-5-[1,4,5,6-四氢-4-(羟甲基)-6-羰基-3-哒嗪基]-
苯甲曲秦
苯甲吗啉酮
苯基2-(2-苯基吗啉-4-基)乙基碳酸酯盐酸盐
苯二甲吗啉一氢酒石酸盐
苯二甲吗啉
苯乙酮 O-(吗啉基羰基甲基)肟
芬美曲秦
芬布酯盐酸盐
芬布酯
脾脏酪氨酸激酶(SYK)抑制剂
脱氯利伐沙班
脱氟雷奈佐利
羟基1-(3-氯苯基)-2-[(1,1-二甲基乙基)氨基]-1-丙酮盐酸盐
福沙匹坦苄酯
福沙匹坦杂质26
福沙匹坦N-苄基杂质
福曲他明
碘化N-甲基丙基吗啉
碘化N-甲基,乙基吗啉
硝酸吗啉