N-(1-morpholin-4-yl-1-benzyl)acetamide | 37733-80-5
中文名称
——
中文别名
——
英文名称
N-(1-morpholin-4-yl-1-benzyl)acetamide
英文别名
N-[morpholino(phenyl)methyl]acetamide;N-(1-morpholinobenzyl)acetamide;N-[1-morpholinobenzyl]acetamide;morpholinobenzyl acetamide;MBA;N-(morpholin-4-yl-phenyl-methyl)-acetamide;N-[morpholin-4-yl(phenyl)methyl]acetamide
CAS
37733-80-5
化学式
C13H18N2O2
mdl
——
分子量
234.298
InChiKey
LMMMOBNNWPVEGZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:168 °C(Solv: ethanol (64-17-5))
-
沸点:414.3±45.0 °C(Predicted)
-
密度:1.134±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.46
-
拓扑面积:41.6
-
氢给体数:1
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,4'-苯亚甲基二吗啉 4,4'-(phenylmethylene)bismorpholine 6425-08-7 C15H22N2O2 262.352
反应信息
-
作为反应物:描述:4-氨基安替比林 、 N-(1-morpholin-4-yl-1-benzyl)acetamide 以 乙醇 为溶剂, 反应 2.0h, 以68%的产率得到N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-N'-(morpholin-4-yl-phenyl-methyl)-acetamidine参考文献:名称:Raman; Ravichandran, Polish Journal of Chemistry, 2005, vol. 79, # 7, p. 1107 - 1114摘要:DOI:
-
作为产物:描述:乙酰胺 、 4,4'-苯亚甲基二吗啉 反应 0.12h, 以56%的产率得到N-(1-morpholin-4-yl-1-benzyl)acetamide参考文献:名称:Solvent-free synthesis of monoacylaminals from the reaction of amides and aminals as precursors in carbinolamide synthesis摘要:A solvent-free method of generating monoacylaminals by heating the amide and aminal starting materials in the presence of one another has been developed. Yields were generally between 45% and 65% with the monoacylaminal being isolated, needing no further purification after drying under high vacuum. (C) 2010 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2010.09.043
文献信息
-
[EN] ANTIMICROBIAL AGENTS<br/>[FR] AGENTS ANTI-MICROBIENS申请人:UNIV RUTGERS公开号:WO2013142712A1公开(公告)日:2013-09-26The invention provides compounds of formual (I): wherein R1-R7 and W have any of the values defined in the specification, and salts thereof. The compounds have good solubility and are useful for treating bacterial infections.该发明提供了式(I)的化合物:其中R1-R7和W具有规范中定义的任何值,以及其盐。这些化合物具有良好的溶解性,并可用于治疗细菌感染。
-
Synthesis, Characterization and Antimicrobial Studies of a New Mannich Base<i>N</i>-[Morpholino(phenyl)methyl]acetamide and Its Cobalt(II), Nickel(II) and Copper(II) Metal Complexes作者:L. Muruganandam、K. KrishnakumarDOI:10.1155/2012/767941日期:——
A new Mannich base
N -[morpholino(phenyl)methyl]acetamide (MBA), was synthesized and characterized by spectral studies. Chelates of MBA with cobalt(II), nickel(II) and copper(II) ions were prepared and characterized by elemental analyses, IR and UV spectral studies. MBA was found to act as a bidentate ligand, bonding through the carbonyl oxygen of acetamide group and CNC nitrogen of morpholine moiety in all the complexes. Based on the magnetic moment values and UV-Visible spectral data, tetracoordinate geometry for nitrato complexes and hexacoordinate geometry for sulphato complexes were assigned. The antimicrobial studies show that the Co(II) nitrato complex is more active than the other complexes. -
Peroxo complexes of uranium(VI) containing nitrogen and oxygen donor ligands作者:Balgar Singh、Simpy Mahajan、H. N. Sheikh、Mohita Sharma、Bansi Lal KalsotraDOI:10.1134/s003602361208013x日期:2012.8The uranium(VI) peroxo complexes containing Mannich base ligands having composition [UO(O-2)L-L(NO3)(2)] where L-L = morpholinobenzyl acetamide (MBA), piperidinobenzyl acetamide (PBA), morpholinobenzyl benzamide (MBB), piperidinobenzyl benzamide (PBB), morpholinomethyl benzamide (MMB), piperidinomethyl benzamide (PMB), morpholinobenzyl formamide (MBF)}, piperidinobenzyl formamide (PBF) are reported. In a typical reaction UO2(NO3)(2) center dot 6H(2)O (1 mmol, 0.502 g) was dissolved in methanol. An equimolar (1 mmol) methanolic solution (30 mL) of the ligand (Mannich bases) was added to a solution of uranyl nitrate followed by addition of potassium hydroxide (KOH) (2 mmol, 0.1122 g). The solution was refluxed for 15 min and then 10 mL of 30% hydrogen peroxide (H2O2) was added dropwise and was refluxed for an additional 1 h. The synthesized complexes have been characterized by various physico-chemical techniques, viz. elemental analysis, molar conductivity, magnetic susceptibility measurements, infra red, electronic, mass spectral and TGA/DTA studies. These studies revealed that the synthesized complexes are non-electrolytic and diamagnetic in nature. The ligands are bound to metal in a bidentate mode through carbonyl oxygen and the ring nitrogen. Thermal analysis result provides conclusive evidence for the absence of water molecule in the complexes. Mass spectra confirm the molecular mass of the complexes. Antibacterial activity of complexes revealed enhanced activity of complexes as compared to corresponding free ligands. Molecular modeling suggests pentagonal bipyramidal structure for complexes.
-
FLOCH Y. LE; PLUSQUELLEC D.; SOYER N.; KERFANTO M., BULL. SOC. CHIM. FRANCE, PART 2, 1979, NO 7-8, 409-414作者:FLOCH Y. LE、 PLUSQUELLEC D.、 SOYER N.、 KERFANTO M.DOI:——日期:——
-
ANTIMICROBIAL AGENTS申请人:Rutgers, the State University of New Jersey公开号:US20150031694A1公开(公告)日:2015-01-29The invention provides compounds of formula (I): wherein R 1 -R 7 and W have any of the values defined in the specification, and salts thereof. The compounds have good solubility and are useful for treating bacterial infections.
表征谱图
-
氢谱1HNMR
-
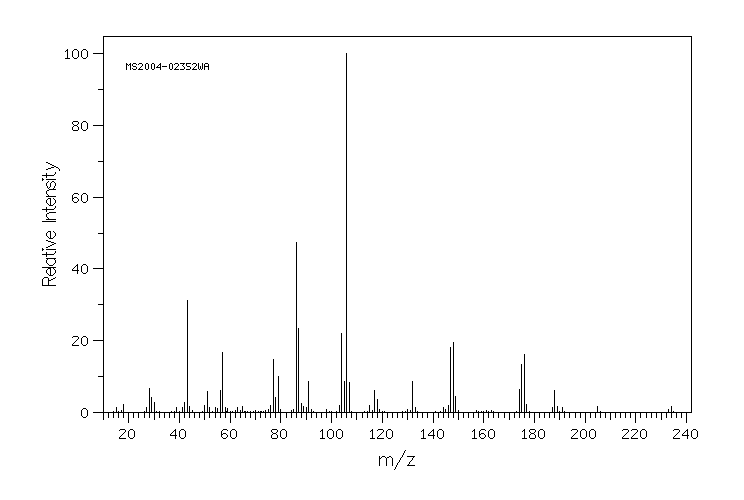
质谱MS
-
碳谱13CNMR
-
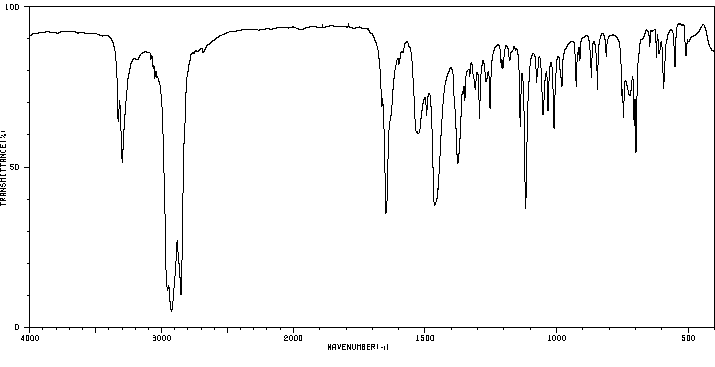
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4-甲基环戊-1-烯-1-基)(吗啉-4-基)甲酮
(2-肟基-氰基乙酸乙酯)-N,N-二甲基-吗啉基脲六氟磷酸酯
鲸蜡基乙基吗啉氮鎓乙基硫酸盐
马啉乙磺酸钾
预分散OTOS-80
顺式4-(氮杂环丁烷-3-基)-2,2-二甲基吗啉
顺式-N-亚硝基-2,6-二甲基吗啉
顺式-3,5-二甲基吗啉
顺-2,6-二甲基-4-(4-硝基苯基)吗啉
非屈酯
雷奈佐利二聚体
阿瑞杂质9
阿瑞杂质12
阿瑞吡坦磷的二卞酯
阿瑞吡坦杂质
阿瑞吡坦杂质
阿瑞吡坦EP杂质C
阿瑞吡坦
阿瑞吡坦
阿瑞匹坦非对映异构体2R3R1R
阿瑞匹坦杂质A异构体
阿瑞匹坦杂质54
阿瑞匹坦-M3代谢物
钾[2 - (吗啉- 4 -基)乙氧基]甲基三氟硼酸
酮康唑杂质
邻苯二甲酸单吗啉
调节安
试剂2-(4-Morpholino)ethyl2-bromoisobutyrate
茂硫磷
苯甲腈,2-(4-吗啉基)-5-[1,4,5,6-四氢-4-(羟甲基)-6-羰基-3-哒嗪基]-
苯甲曲秦
苯甲吗啉酮
苯基2-(2-苯基吗啉-4-基)乙基碳酸酯盐酸盐
苯二甲吗啉一氢酒石酸盐
苯二甲吗啉
苯乙酮 O-(吗啉基羰基甲基)肟
芬美曲秦
芬布酯盐酸盐
芬布酯
脾脏酪氨酸激酶(SYK)抑制剂
脱氯利伐沙班
脱氟雷奈佐利
羟基1-(3-氯苯基)-2-[(1,1-二甲基乙基)氨基]-1-丙酮盐酸盐
福沙匹坦苄酯
福沙匹坦杂质26
福沙匹坦N-苄基杂质
福曲他明
碘化N-甲基丙基吗啉
碘化N-甲基,乙基吗啉
硝酸吗啉