N-(furan-2-ylmethyl)-N-methylaniline | 105909-65-7
中文名称
——
中文别名
——
英文名称
N-(furan-2-ylmethyl)-N-methylaniline
英文别名
N-Furfuryl-N-methylaniline
CAS
105909-65-7
化学式
C12H13NO
mdl
——
分子量
187.241
InChiKey
PUFNOLHEVROYKO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:142-145 °C(Press: 10 Torr)
-
密度:1.095±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:16.4
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-糠基苯胺 N-(furan-2-ylmethyl)aniline 4439-56-9 C11H11NO 173.214
反应信息
-
作为反应物:描述:N-(furan-2-ylmethyl)-N-methylaniline 在 氘代二甲亚砜 、 potassium tert-butylate 作用下, 反应 1.0h, 以85%的产率得到N-(furan-2-yl-5-d1-methyl-d2)-N-methylaniline参考文献:名称:KO t -Bu / DMSO- d 6方便地合成氘标记的胺和氮杂环摘要:芳基甲胺和氮杂环的H / D交换可通过KO t -Bu / DMSO- d 6方便地实现。该方法也适用于苯基苄基醚,二芳基甲烷和烷基芳烃。这些H / D交换反应被建议通过自由基途径进行。DOI:10.1016/j.tet.2015.01.015
-
作为产物:描述:L-(+)-arabinose 在 二甲基苯基硅烷 、 per-rhenic acid 作用下, 以 1,4-二氧六环 为溶剂, 反应 3.0h, 生成 N-(furan-2-ylmethyl)-N-methylaniline参考文献:名称:HReO 4催化从生物质资源一锅法合成胺摘要:这项工作描述了由HReO 4催化的碳水化合物一锅法合成糠胺。这些一锅三反应法和四反应法可分别将木糖和木聚糖转化为多种仲胺和叔胺,并具有良好的总收率和化学选择性。DOI:10.1039/c8gc00915e
文献信息
-
Synthesis of tertiary arylamines: Lewis acid-catalyzed direct reductive N-alkylation of secondary amines with ketones through an alternative pathway作者:Onkar S. Nayal、Maheshwar S. Thakur、Vinod Bhatt、Manoranjan Kumar、Neeraj Kumar、Bikram Singh、Upendra SharmaDOI:10.1039/c6cc04381j日期:——We report herein a highly efficient, tin(II)/PMHS catalyzed reductive N-alkylation of arylamines with ketones affording tertiary arylamines. Very wide substrate scope was observed for current catalytic method as all six...我们在此报告了一种高效的锡(II)/ PMHS催化的芳基胺与酮的还原性N-烷基化反应,提供了叔芳基胺。当前的催化方法观察到非常宽的底物范围,因为所有六个...
-
Ligand-free Iron(II)-Catalyzed N-Alkylation of Hindered Secondary Arylamines with Non-activated Secondary and Primary Alcohols <i>via</i> a Carbocationic Pathway作者:Onkar S. Nayal、Maheshwar S. Thakur、Manoranjan Kumar、Neeraj Kumar、Sushil K. MauryaDOI:10.1002/adsc.201701183日期:2018.2.15Secondary benzylic alcohols represent a challenging class of substrates for N‐alkylation of amines. Herein, we describe an iron(II)‐catalyzed eco‐friendly protocol for N‐alkylation of secondary arylamines with secondary benzyl alcohols through a carbocationic pathway instead of the known borrowing hydrogen transfer (BHT) approach. Transiently generated carbocations, produced from alcohols via self‐condensation
-
BF<sub>3</sub>·Et<sub>2</sub>O as a metal-free catalyst for direct reductive amination of aldehydes with amines using formic acid as a reductant作者:Zhenli Luo、Yixiao Pan、Zhen Yao、Ji Yang、Xin Zhang、Xintong Liu、Lijin Xu、Qing-Hua FanDOI:10.1039/d1gc01468d日期:——direct reductive amination of aldehydes with amines using formic acid as a reductant under the catalysis of inexpensive BF3·Et2O has been developed. A wide range of primary and secondary amines and diversely substituted aldehydes are compatible with this transformation, allowing facile access to various secondary and tertiary amines in high yields with wide functional group tolerance. Moreover, the
-
Direct <i>N‐</i> Alkylation/Fluoroalkylation of Amines Using Carboxylic Acids via Transition‐Metal‐Free Catalysis作者:Chunlei Lu、Zetian Qiu、Maojie Xuan、Yan Huang、Yongjia Lou、Yiling Zhu、Hao Shen、Bo‐Lin LinDOI:10.1002/adsc.202000679日期:2020.10.6scalable protocol of direct N‐mono/di‐alkyl/fluoroalkylation of primary/secondary amines has been constructed with various carboxylic acids as coupling agents under the catalysis of a simple air‐tolerant inorganic salt, K3PO4. Advantageous features include 100 examples, 10 drugs and drug‐like amines, fluorinated complex tertiary amines, gram‐scale synthesis and isotope‐labelling amine, thus demonstrating
-
Efficient Ruthenium(II)-Catalyzed Direct Reductive Amination of Aldehydes under Mild Conditions Using Hydrosilane as the Reductant作者:Bin Li、Yibiao Li、Jianxiong Zheng、Weifeng Zeng、Lu ChenDOI:10.1055/s-0036-1588092日期:——also report an interesting direductive amination of 2-ethylbutanal. A direct reductive amination of aldehydes with anilines is performed with a ruthenium(II)-(arene) catalyst. The [RuCl2(p-cymene)]2/Ph2SiH2 catalytic system is very efficient for the synthesis of secondary amines and tertiary amines in good yields, and is highly chemoselective, tolerating a wide range of functional groups, such as NO2摘要 用钌(II)-(芳烃)催化剂进行醛与苯胺的直接还原胺化。[RuCl 2(p- cymene)] 2 / Ph 2 SiH 2催化体系对于高效率地合成仲胺和叔胺非常有效,并且具有高度的化学选择性,可以耐受各种官能团,例如NO 2,CN,CO 2 Me,F,Cl,Br,OMe,Me,呋喃基和烷基。我们还报告了一个有趣的2-乙基丁醛二还原胺化反应。 用钌(II)-(芳烃)催化剂进行醛与苯胺的直接还原胺化。[RuCl 2(p- cymene)] 2 / Ph 2 SiH 2催化体系对于高效率地合成仲胺和叔胺非常有效,并且具有高度的化学选择性,可以耐受各种官能团,例如NO 2,CN,CO 2 Me,F,Cl,Br,OMe,Me,呋喃基和烷基。我们还报告了一个有趣的2-乙基丁醛二还原胺化反应。
表征谱图
-
氢谱1HNMR
-
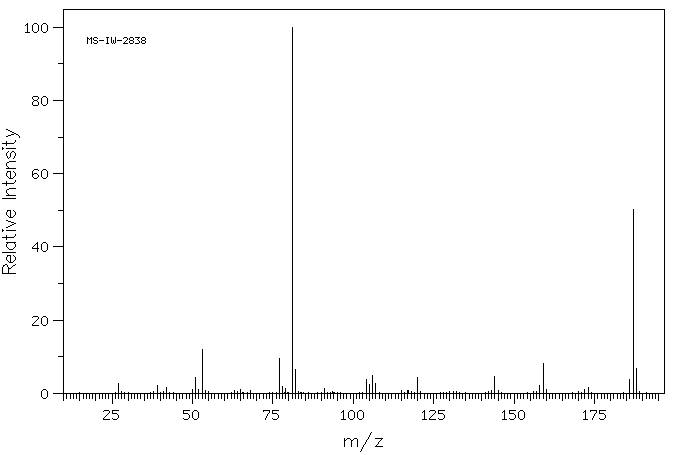
质谱MS
-
碳谱13CNMR
-
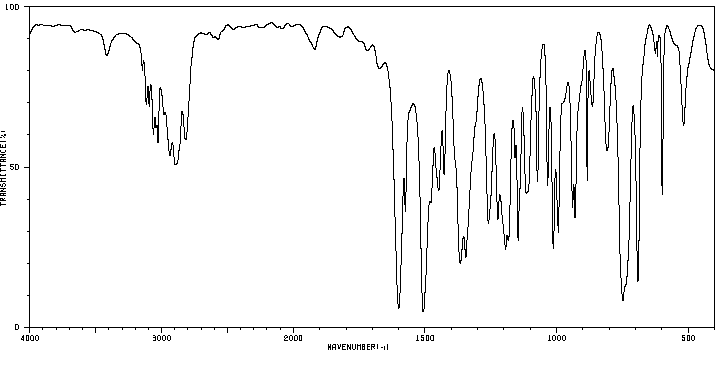
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷