2-[(1E)2-phenylethenyl]-4,5-dihydro-1,3-thiazole | 27565-37-3
中文名称
——
中文别名
——
英文名称
2-[(1E)2-phenylethenyl]-4,5-dihydro-1,3-thiazole
英文别名
(E)-2-[2-phenylethenyl]-4,5-dihydro-1,3-thiazole;4,5-dihydro-2-[(E)-phenylethenyl]-1,3-thiazole;(E)-2-styryl-4,5-dihydrothiazole;2-trans-styryl-4,5-dihydro-thiazole;2-trans-Styryl-4,5-dihydro-thiazol;2-[(E)-2-phenylethenyl]-4,5-dihydro-1,3-thiazole
CAS
27565-37-3
化学式
C11H11NS
mdl
——
分子量
189.281
InChiKey
BHXRULBFMDQOMX-VOTSOKGWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:37.7
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:参考文献:名称:烯基噻唑啉的非对映选择性二聚化:综合合成和计算研究摘要:分别用三氯乙酰氯和三氟乙酸酐处理后,2-烯基-1,3-噻唑啉 1 的酰化二聚反应得到化合物 3 和 8。该反应是完全非对映选择性的,特别是只产生一个双键异构体。对反应的范围进行了综合评估,而计算研究阐明了反应的机制和立体控制的起源。(© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2005)DOI:10.1002/ejoc.200500360
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 tetraphosphorus decasulfide 作用下, 生成 2-[(1E)2-phenylethenyl]-4,5-dihydro-1,3-thiazole参考文献:名称:Ballard et al., Chemistry of Penicillin摘要:DOI:
文献信息
-
Microwave-Assisted Synthesis of 2-Substituted 2-Thiazolines and 5,6-Dihydro-4H-1,3-thiazines作者:Liliana R. Orelli、María C. Mollo、Juan A. Bisceglia、Natalia B. Kilimciler、Michele MancinelliDOI:10.1055/s-0039-1690822日期:2020.6An efficient and general method for the synthesis of 2-substituted thiazolines and 5,6-dihydro-4H-1,3-thiazines is developed via microwave-assisted ring closure of ω-thioamidoalcohols promoted by ethyl polyphosphate (PPE). The cyclization reaction involves an SN2-type mechanism and features the advantages of very short reaction times, high yields and a predictable stereochemical outcome. The acyclic
-
Facile Syntheses of Oxazolines and Thiazolines with <i>N</i>-Acylbenzotriazoles under Microwave Irradiation作者:Alan R. Katritzky、Chunming Cai、Kazuyuki Suzuki、Sandeep K. SinghDOI:10.1021/jo0355092日期:2004.2.1Microwave reactions of 2-amino-2-methyl-1-propanol (2) or 2-aminoethanethiol hydrochloride (4) with readily available N-acylbenzotriazoles 1a−j in the presence of SOCl2 produced 2-substituted 2-oxazolines 3a−j in 84−98% yields and 2-substituted thiazolines 5a−i in 85−97% yields, respectively. With use of this method chiral oxazoline 6, bisoxazoline 7, bisthiazoline 8, and 5,6-dihydro-4H-1,3-oxazines
-
A one step synthesis of thiazolines from esters作者:Carl A. Busacca、Yong Dong、Earl M. SpinelliDOI:10.1016/0040-4039(96)00451-0日期:1996.4Condensation of the triisobutylaluminum complex of cysteamine HCl with a variety of carboxylic esters furnishes thiazolines in one step. This new method has been applied to chiral α-aminoesters to give α-amino thiazolines of high optical purity, yet N-benzyl protection of the amine is required.
-
Asymmetric Hetero-Diels-Alder Reactions with Heterocumulenes作者:Mark C. Elliott、Alexandra E. Monk、Elbertus Kruiswijk、David E. Hibbs、Robert L. Jenkins、David V. JonesDOI:10.1055/s-1999-2853日期:1999.9Chiral 2-alkenyloxazolines and 2-alkenylthiazolines react with isocyanates and ketenes to give formal hetero-Diels-Alder adducts with complete diastereocontrol. In each case an electron-rich double bond in the adduct is prone to a second addition of the heterocumulene. In the case of the oxazoline adducts the second addition is faster than the first, while with thiazolines the second addition is slower
-
Photostencils for screenprinting申请人:AUTOTYPE INTERNATIONAL LIMITED公开号:EP0313221A2公开(公告)日:1989-04-26Photodimerisable polymer compositions are based upon derivatives of polyvinylalcohol and constituents of the formula: wherein m = 0 or 1; n = 0 or an integer from 1-6; subject to the proviso that when n = 0, then m = 0; b = H or a tertiary amine, methoxy or ethoxy group; X = 0, S or Se or an NR₁ or C(R₁)₂ group, wherein R₁ = H or an alkyl, hydroxyalkyl, aryl or aralkyl group, Z = an anion and wherein R₂ and R₃ are the same or different and each represents H, halogen or an alkyl, hydroxyalkyl, aryl, nitro or cyano group or R₂ and R₃ taken together may form part of the same aromatic or aliphatic ring system. The preparation and typical uses of the polymer compositions as photostencils for screenprinting are also described.
表征谱图
-
氢谱1HNMR
-
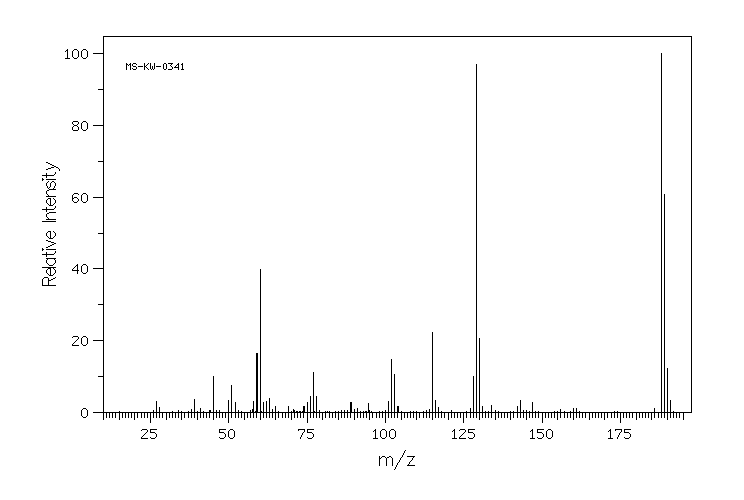
质谱MS
-
碳谱13CNMR
-
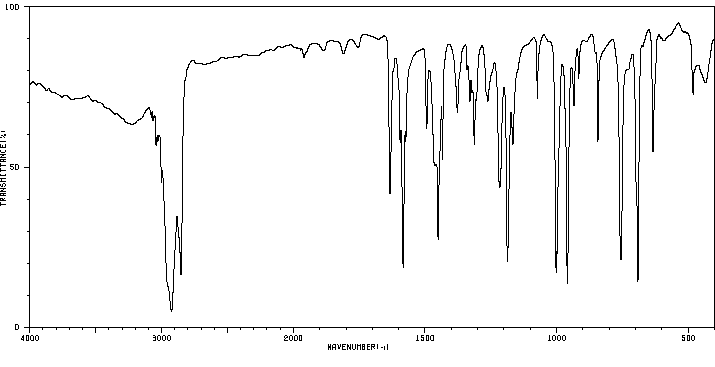
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫