(E)-cinnamic N-phenyl carbamate | 28315-20-0
中文名称
——
中文别名
——
英文名称
(E)-cinnamic N-phenyl carbamate
英文别名
cinnamyl phenylcarbamate;phenylcarbamic acid trans-cinnamyl ester;Phenylcarbamidsaeure-trans-cinnamylester;Cinnamyl carbanilate;[(E)-3-phenylprop-2-enyl] N-phenylcarbamate
CAS
28315-20-0
化学式
C16H15NO2
mdl
——
分子量
253.301
InChiKey
DXODDGVNDLOMLO-JXMROGBWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:375.8±31.0 °C(Predicted)
-
密度:1.182±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:19
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:2
反应信息
-
作为反应物:描述:(E)-cinnamic N-phenyl carbamate 在 仲丁基锂 、 sodium hydride 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 、 mineral oil 为溶剂, 反应 15.5h, 生成 ω-苄基苯乙酮 、 (Z)-1,3-diphenylprop-1-enyl methylcarbamate参考文献:名称:锂编排:氨基甲酸酯稳定的碳负离子的分子内芳基化及其机理的原位红外光谱和DFT计算摘要:在锂抗衡离子存在下,O-烯丙基,O-炔丙基或O-苄基氨基甲酸酯的去质子化反应可生成氨基甲酸酯稳定的有机锂化合物,可将其用亲电子试剂猝灭。现在我们报告说,当烯丙基,炔丙基或苄基氨基甲酸酯带有一个N-芳基取代基时,发生芳基迁移,从而导致立体化学转化和氨基甲酸酯α到氧的C-芳基化。芳基迁移是分子内S N尽管缺乏阴离子稳定的芳基取代基,但仍会发生Ar反应。我们的原位红外研究揭示了重排途径中的许多中间体,包括“预锂化复合物”,去质子化的氨基甲酸酯,重排的阴离子以及最终的芳基化氨基甲酸酯。在芳基迁移过程中未获得脱芳香化中间体的证据。DFT计算预测,在反应过程中,溶剂化的Li阳离子会从碳负离子中心移出,从而释放出孤对,从而对远端苯环进行亲核攻击。这种电荷分离导致几种可供选择的构象。有李+的人结合到氨基甲酸酯上的氧产生最低能级的跃迁结构,并且还导致构型的反转。与IR研究一致,DFT计算未能找到脱芳烃的中间体。DOI:10.1002/chem.201201761
-
作为产物:描述:参考文献:名称:锂编排:氨基甲酸酯稳定的碳负离子的分子内芳基化及其机理的原位红外光谱和DFT计算摘要:在锂抗衡离子存在下,O-烯丙基,O-炔丙基或O-苄基氨基甲酸酯的去质子化反应可生成氨基甲酸酯稳定的有机锂化合物,可将其用亲电子试剂猝灭。现在我们报告说,当烯丙基,炔丙基或苄基氨基甲酸酯带有一个N-芳基取代基时,发生芳基迁移,从而导致立体化学转化和氨基甲酸酯α到氧的C-芳基化。芳基迁移是分子内S N尽管缺乏阴离子稳定的芳基取代基,但仍会发生Ar反应。我们的原位红外研究揭示了重排途径中的许多中间体,包括“预锂化复合物”,去质子化的氨基甲酸酯,重排的阴离子以及最终的芳基化氨基甲酸酯。在芳基迁移过程中未获得脱芳香化中间体的证据。DFT计算预测,在反应过程中,溶剂化的Li阳离子会从碳负离子中心移出,从而释放出孤对,从而对远端苯环进行亲核攻击。这种电荷分离导致几种可供选择的构象。有李+的人结合到氨基甲酸酯上的氧产生最低能级的跃迁结构,并且还导致构型的反转。与IR研究一致,DFT计算未能找到脱芳烃的中间体。DOI:10.1002/chem.201201761
文献信息
-
Practical Synthesis of Allylic Silanes from Allylic Esters and Carbamates by Stereoselective Copper-Catalyzed Allylic Substitution Reactions作者:Martin Oestreich、Gertrud AuerDOI:10.1002/adsc.200404381日期:2005.4The first copper-catalyzed allylic substitution reactions of allylic acetates and carbamates employing a bis(triorganosilyl)zinc reagent are reported. This novel procedure avoids the use of stoichiometric amounts of copper salts which are usually mandatory with this chemistry. Application of this methodology to standard model substrates substantiates a high diastereoselectivity for the double bond
-
Expedient access to branched allylic silanes by copper-catalysed allylic substitution of linear allylic halides作者:Devendra J. Vyas、Martin OestreichDOI:10.1039/b920793g日期:——An unprecedented copper-catalysed allylic transposition enables the regioselective synthesis of branched allylic silanes from linear allylic halides through direct C-Si bond formation.
-
Silicon Grignard Reagents as Nucleophiles in Transition-Metal-Catalyzed Allylic Substitution作者:Weichao Xue、Martin OestreichDOI:10.1055/s-0037-1610309日期:2019.1shown to promote allylic substitution reactions of allylic electrophiles with silicon Grignard reagents. The procedure was further elaborated for CuI as catalyst. The regioselectively is independent of the leaving group for primary allylic precursors, favoring α over γ. The stereochemical course of this allylic transposition was probed with a cyclic system, and anti-diastereoselectivity was obtained.作为《五十周年综合报告》的一部分发行-黄金周年纪念日 抽象的 已显示出广泛的过渡金属催化剂可促进烯丙基亲电试剂与硅格氏试剂的烯丙基取代反应。对于CuI作为催化剂,该程序进一步进行了详细说明。区域选择性与伯烯丙基前体的离去基团无关,与α相比,α更有利。此烯丙基换位的立体化学过程,用一个环状系统探测,和抗获得-diastereoselectivity。 已显示出广泛的过渡金属催化剂可促进烯丙基亲电试剂与硅格氏试剂的烯丙基取代反应。对于CuI作为催化剂,该程序进一步进行了详细说明。区域选择性与伯烯丙基前体的离去基团无关,与α相比,α更有利。此烯丙基换位的立体化学过程,用一个环状系统探测,和抗获得-diastereoselectivity。
-
Copper-Catalyzed SiB Bond Activation in Branched-Selective Allylic Substitution of Linear Allylic Chlorides作者:Devendra J. Vyas、Martin OestreichDOI:10.1002/anie.201004658日期:2010.11.2The harder they come, the better they leave! Chloride is superior to all common leaving groups in the γ‐selective allylic substitution of linear precursors. This approach involves a novel copper‐catalyzed SiB bond activation (see scheme; γ/α≥98:2, 7 examples; Si=SiMe2Ph and B=Bpin with pin=pinacolato).
-
Regio- and diastereoselective Pd-catalyzed aminochlorocyclization of allylic carbamates: scope, derivatization, and mechanism作者:Bruna Papa Spadafora、Francisco Wanderson Moreira Ribeiro、Jullyane Emi Matsushima、Elaine Miho Ariga、Isaac Omari、Priscila Machado Arruda Soares、Diogo de Oliveira-Silva、Elisângela Vinhato、J. Scott McIndoe、Thiago Carita Correra、Alessandro RodriguesDOI:10.1039/d1ob00670c日期:——diastereoselective synthesis of oxazolidinones via a Pd-catalyzed vicinal C–N/C–Cl bond-forming reaction from internal alkenes of allylic carbamates is reported. The oxazolidinones are obtained in yields of 44 to 95% with high to excellent diastereoselectivities (from 6 : 1 to >20 : 1 dr) from readily available precursors. This process is scalable, and the products are suitable for the synthesis of useful amino
表征谱图
-
氢谱1HNMR
-
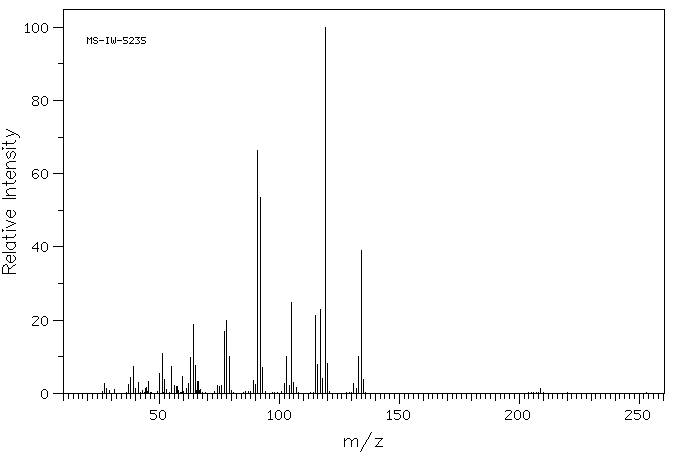
质谱MS
-
碳谱13CNMR
-
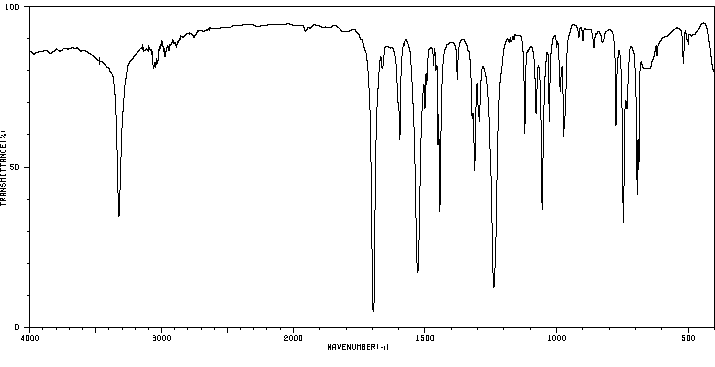
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫