(E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione | 5465-37-2
中文名称
——
中文别名
——
英文名称
(E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione
英文别名
——
CAS
5465-37-2
化学式
C16H10Cl2O2
mdl
——
分子量
305.16
InChiKey
NOWZMUNUVDBDQQ-MDZDMXLPSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:170 °C
-
沸点:443.7±45.0 °C(Predicted)
-
密度:1.321±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:20
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (Z)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione 27297-60-5 C16H10Cl2O2 305.16 对氯苯乙酮 para-chloroacetophenone 99-91-2 C8H7ClO 154.596 2'-溴-4-氯苯乙酮 4-Chlorophenacyl bromide 536-38-9 C8H6BrClO 233.492 —— 1-(4-chlorophenyl)-2-diazoethanone 3282-33-5 C8H5ClN2O 180.593
反应信息
-
作为反应物:描述:(E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione 在 aluminum isopropoxide 、 异丙醇 作用下, 生成 1,4-二(4-氯苯基)丁烷-1,4-二酮参考文献:名称:减少 1 : 4-二酮摘要:二苯甲酰乙烯在某些条件下用金属组合还原或催化氢化产生相应的二苯甲酰基,并且有证据表明该反应通过氢的 1:6-加成和中间体二烯醇的快速酮化发生。Lutz 和 Reveley2 显示了一种这样的二烯醇的存在,但是没有将其分离:需要确认这种机制,特别是因为 1:6 加成相对不常见。我们已经通过在异丙醇中用异丙醇铝还原几个取代的二苯甲酰亚乙基来实现这一点,异丙醇是一种已知的试剂,可以攻击羰基,同时保持烯键完整。还原产物总是相应的二苯甲酰,通过与真实样品的混合熔点进行鉴定(色谱吸附后)。DOI:10.1038/161054a0
-
作为产物:描述:对氯苯乙酮 在 碘 、 copper(ll) bromide 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 以33%的产率得到(E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione参考文献:名称:通过电子给体-受体配合物对烷基苯胺和二苯甲酰基乙烯的可见光驱动立体选择性环化摘要:提出了烯烃与N,N-取代的二烷基苯胺之间无催化剂,立体选择性可见光驱动的环合反应,用于合成取代的四氢喹啉。该反应是由电子给体-受体(EDA)络合物的光激发而驱动的,所获得的产物以良好的收率和高收率获得,具有完全的非对映选择性。提供了EDA-复合物的机理原理和光化学表征。DOI:10.1021/acs.joc.0c02819
文献信息
-
[1+1+1] Cyclotrimerization for the Synthesis of Cyclopropanes作者:Srimanta Manna、Andrey P. AntonchickDOI:10.1002/anie.201600807日期:2016.4.18The synthesis of small rings by functionalization of C(sp(3) )-H bonds remains a great challenge. We report for the first time a copper-catalyzed [1+1+1] cyclotrimerization of acetophenone derivatives under mild reaction conditions. The reaction has a broad scope for the stereoselective synthesis of cyclopropanes by trimerization of acetophenone. The developed transformation is based on an extraordinary
-
Copper‐Catalyzed (2+1) Annulation of Acetophenones with Maleimides: Direct Synthesis of Cyclopropanes作者:Srimanta Manna、Andrey P. AntonchickDOI:10.1002/anie.201502872日期:2015.12A practical copper‐catalyzed direct oxidative cyclopropanation of electron‐deficient alkenes with acetophenone derivatives is reported. The dehydrogenative annulation involves a double CH bond functionalization at the α‐position of the ketone using di‐tert‐butyl peroxide as oxidant. The broad scope of the reaction and excellent functional‐group tolerance is demonstrated for the stereoselective synthesis
-
Asymmetric Diastereoselective Synthesis of Spirocyclopropane Derivatives of Oxindole作者:Maksim Ošeka、Artur Noole、Sergei Žari、Mario Öeren、Ivar Järving、Margus Lopp、Tõnis KangerDOI:10.1002/ejoc.201402061日期:2014.6A new asymmetric organocatalytic synthesis of spirocyclopropane oxindoles has been developed. The method is based on the Michael addition of N-Boc-protected 3-chlorooxindole to unsaturated 1,4-dicarbonyl compounds, affording trans-substituted spirocyclopropane oxindole derivatives in high diastereo- and enantioselectivity.
-
Lewis Acid-Catalyzed One-Pot, Three-Component Route to Chiral 3,3‘-Bipyrroles作者:Sumit Dey、Churala Pal、Debkumar Nandi、Venkatachalam Sesha Giri、Marek Zaidlewicz、Marek Krzeminski、Lidia Smentek、B. Andes Hess,、Jacek Gawronski、Marcin Kwit、N. Jagadeesh Babu、Ashwini Nangia、Parasuraman JaisankarDOI:10.1021/ol800115p日期:2008.4.13-dicarbonyls 2 using ammonium acetate as a nitrogen source. The axial chirality of bipyrrole was anticipated from the X-ray crystal structure and DFT calculations and confirmed by separating the racemates on a chiral column and subsequent CD spectra of the enantiomers. The absolute configuration of the enantiomers was achieved by theoretical CD spectra calculation using the ZINDO method.
-
Visible-Light-Mediated Oxidative Dimerization of Arylalkynes in the Open Air: Stereoselective Synthesis of (<i>Z</i>)-1,4-Enediones作者:Donglei Wei、Fushun LiangDOI:10.1021/acs.orglett.6b02926日期:2016.11.18An organic photoredox catalytic one-pot protocol is developed for the highly stereoselective synthesis of (Z)-1,4-enediones. The reaction starts directly from alkyne precursors, using 4-(4-cyanophenyl)-2,6-diphenylpyrylium tetrafluoroborate (CN-TPT) as an efficient photosensitizer and dioxygen in the air as a green oxidant. A Csp–Csp oxidative coupling/[4 + 2] cyclization (with dioxygen)/fragmentive
表征谱图
-
氢谱1HNMR
-
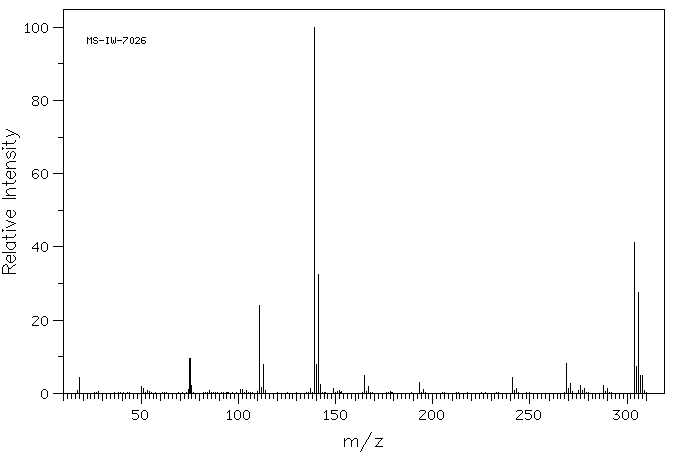
质谱MS
-
碳谱13CNMR
-
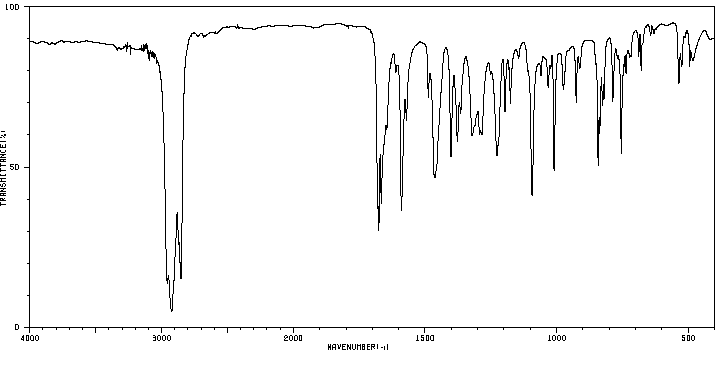
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫