meso-2,3-dichlorobutane | 4028-56-2
中文名称
——
中文别名
——
英文名称
meso-2,3-dichlorobutane
英文别名
2-SR,3-RS-2,3-dichlorobutane;meso 2,3-dichlorobutane;meso-2,3-dichloro-butane;meso-2,3-Dichlor-butan, niedrigersiedende Form;meso-2,3-Dichlor-butan;(R*,S*)-2,3-dichloro-butane;Butane, 2,3-dichloro-, meso-;(2R,3S)-2,3-dichlorobutane
CAS
4028-56-2
化学式
C4H8Cl2
mdl
——
分子量
127.014
InChiKey
RMISVOPUIFJTEO-ZXZARUISSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-80.4°C
-
沸点:122.28°C (estimate)
-
密度:1.1025
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 氯代仲丁烷 s-butyl chloride 78-86-4 C4H9Cl 92.5685 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2,3-三氯丁烷 erythro-1,2,3-trichlorobutane 18338-40-4 C4H7Cl3 161.459 2,2,3-三氯丁烷 2,2,3-trichloro-butane 10403-60-8 C4H7Cl3 161.459
反应信息
-
作为反应物:描述:参考文献:名称:The Free Radical Addition of Thiolacetic Acid and of Hydrogen Bromide to cis- and trans-2-Chloro-2-butene1摘要:DOI:10.1021/ja01505a021
-
作为产物:描述:参考文献:名称:Morgan; Hickinbottom, Journal of the Chemical Society, 1923, vol. 123, p. 103摘要:DOI:
文献信息
-
Nucleophilic displacement reactions at Se (II)作者:George H. Schmid、Dennis G. GarrattDOI:10.1016/s0040-4020(01)96718-3日期:1985.1The products of the title reaction depend upon the relative concentrations of reactants. With equimolar concentrations or an excess of 2-chloroalkyl phenyl selenide, the products are 1,2-dicloroethane and a diaryldiselenide. When excess areneselenenyl chloride is used ,the products are a diaryl diselenide and 2-chloroalkyl phenyl selenide dichloride. A mechanism involving nucleophilic displacement
-
Novel Desulfitobacterium strain for the degradation of chloroalkenes申请人:UNIVERSITEIT GENT公开号:EP1352977A2公开(公告)日:2003-10-15The present invention relates to a novel species of the Desulfitobacterium genus capable of dehalogenating haloalkanes under anaerobic conditions. The invention relates further to compositions and methods for dehalogenating haloalkanes. The properties of the novel bacterium of the present invention allows applications such as the in situ remediation of anaerobic contaminated sites. Halogenated compounds which can be degraded with the bacteria of the present invention include pollutants such as 1,2-dichloroethane and 1,2-dichloropropane.
-
Lasne,M.-C. et al., Bulletin de la Societe Chimique de France, 1972, # 12, p. 4592 - 4596作者:Lasne,M.-C. et al.DOI:——日期:——
-
Lucas; Simpson; Carter, Journal of the American Chemical Society, 1925, vol. 47, p. 1467作者:Lucas、Simpson、CarterDOI:——日期:——
-
Tischtschenko; Tschurbakow, Zhurnal Obshchei Khimii, 1937, vol. 7, p. 665作者:Tischtschenko、TschurbakowDOI:——日期:——
表征谱图
-
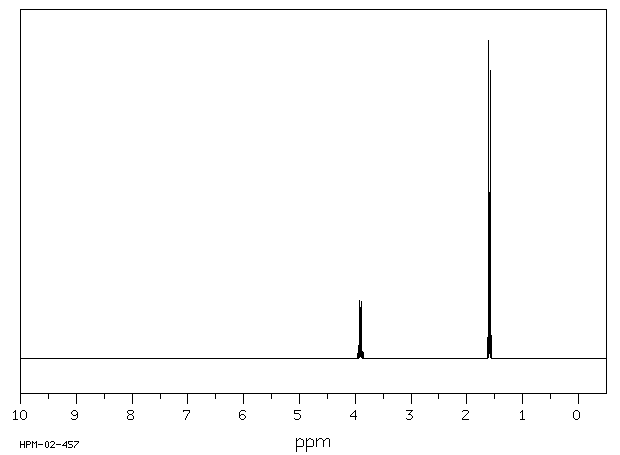
氢谱1HNMR
-
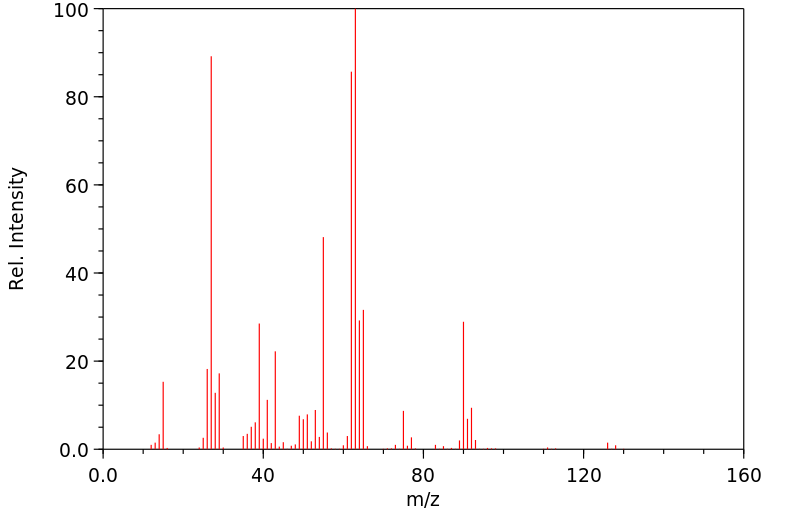
质谱MS
-
碳谱13CNMR
-
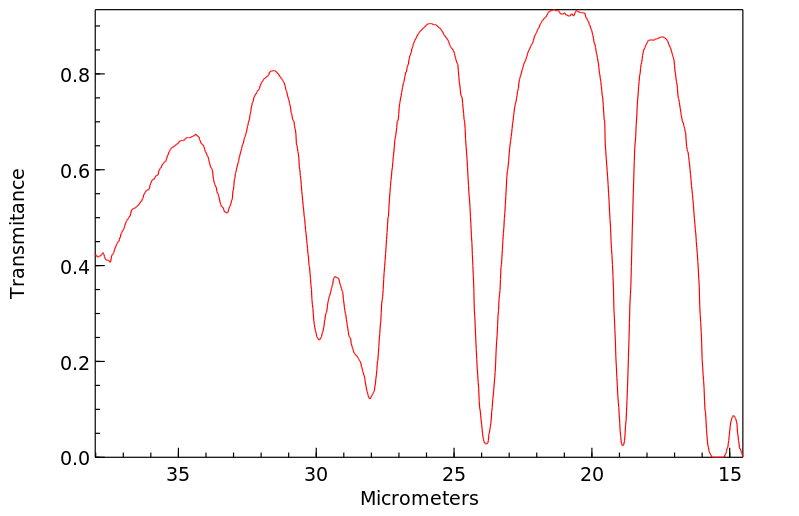
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷