1-(2-methoxyphenoxy)propan-2-one | 6437-46-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1541.1
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
储存条件:室温
SDS
: 1-(2-Methoxyphenoxy)-2-propanone
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
当心 - 物质尚未完全测试。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C10H12O3
分子式
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(2-羟基苯氧基)丙酮 1-(2-hydroxyphenoxy)acetone 13156-23-5 C9H10O3 166.177 愈创甘油醚 guaifenesin 93-14-1 C10H14O4 198.219 2-氯甲基-1,4-苯并二噁烷 2-chloromethyl-1,4-benzodioxane 2164-33-2 C9H9ClO2 184.622 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(2-甲氧基苯氧基)-2-丙胺 1-(2-methoxyphenoxy)propan-2-amine 6505-08-4 C10H15NO2 181.235
反应信息
-
作为反应物:描述:1-(2-methoxyphenoxy)propan-2-one 在 sodium hydroxide 、 lithium aluminium tetrahydride 作用下, 以 乙醚 为溶剂, 生成 3-Hydroxy-2-(2'-methoxyphenoxy)-1-phenyl-1-buten参考文献:名称:Rosnati,V.; Salimbeni,A., Gazzetta Chimica Italiana, 1977, vol. 107, p. 271 - 278摘要:DOI:
-
作为产物:描述:参考文献:名称:Marini-Bettolo et al., Gazzetta Chimica Italiana, 1956, vol. 86, p. 1336,1342摘要:DOI:
文献信息
-
Arylethanolamines derived from salicylamide with .alpha.- and .beta.-adrenoceptor blocking activities. Preparation of labetalol, its enantiomers and related salicylamides作者:James E. Clifton、Ian Collins、Peter Hallett、David Hartley、Lawrence H. C. Lunts、Philip D. WicksDOI:10.1021/jm00348a013日期:1982.6A series of phenethanolamines (3) based on salicylamide has been prepared and shown to possess beta-adrenergic blocking properties. When the basic nitrogen atom was substituted by some aralkyl groups, the compounds also blocked alpha-adrenoceptors. The 1-methyl-3-phenylpropyl derivative labetalol (34) is antihypertensive in animals and man, and syntheses of its four stereoisomers are described. The
-
GC–MS study of thermochemical conversion of guaifenesin in the presence of 1-butyl-3-methylimidazolium-based ionic liquids作者:Sharifah Bee Abd Hamid、Nader Ghaffari Khaligh、Mahdieh Sharifi、Suzaimi JohariDOI:10.1007/s11164-016-2858-3日期:2017.7performed in the presence of 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4] ionic liquid at 80 °C within 2 h. After evaluating the effect of different parameters, such as protic and nonprotic solvents, temperature, reaction time, 1-butyl-3-methylimidazolium-based ionic liquids as process media, and the amount of ionic liquid, the results demonstrate that [BMIM][BF4] facilitates the conversion愈创甘油醚的热化学转化是在80°C下于2小时内在四氟硼酸1-丁基-3-甲基咪唑鎓[BMIM] [BF 4 ]离子液体的存在下进行的。在评估了质子和非质子溶剂,温度,反应时间,基于1-丁基-3-甲基咪唑鎓的离子液体作为工艺介质以及离子液体的量等不同参数的影响后,结果表明[BMIM] [ BF 4 ]促进了愈创甘油醚向3-(2-甲氧基苯氧基)丙醛和其他新产物的转化,而[BMIM] Cl促进了C–O键裂解为2-甲氧基苯酚(愈创木酚)。离子液体[BMIM] [BF 4进行3次使用,而没有任何催化活性的损失。在这项研究中进行了气相色谱-质谱(GC-MS)实验,以揭示愈创甘油醚的降解和产物形成的特征。根据愈创甘油醚转化产物的分布,使用GC-MS数据提出了最合理的机理。
-
SELECTIVE AEROBIC ALCOHOL OXIDATION METHOD FOR CONVERSION OF LIGNIN INTO SIMPLE AROMATIC COMPOUNDS申请人:WISCONSIN ALUMNI RESEARCH FOUNDATION公开号:US20140235838A1公开(公告)日:2014-08-21Described is a method to oxidize lignin or lignin sub-units. The method includes oxidation of secondary benzylic alcohol in the lignin or lignin sub-unit to a corresponding ketone in the presence of unprotected primarily aliphatic alcohol in the lignin or lignin sub-unit. The optimal catalyst system consists of HNO 3 in combination with another Brønsted acid, in the absence of a metal-containing catalyst, thereby yielding a selectively oxidized lignin or lignin sub-unit. The method may be carried out in the presence or absence of additional reagents including TEMPO and TEMPO derivatives.
-
Structure-Activity Relationships in the Domain of Odorants Having Marine Notes作者:Jean-Marc Gaudin、Jean-Yves de Saint LaumerDOI:10.1002/ejoc.201403365日期:2015.3Continuing our investigations into marine note odorants, we herein describe several new scaffolds. Among them, 2,3-dihydrobenzofuran-2-carbaldehyde is particularly interesting. The results demonstrate that the seven-membered ring with a ketone functional group of the Calone 1951® family can be replaced by a five-membered ring carrying an aldehyde function. In addition, this work has allowed us to discover
-
Process for manufacture of aryloxy-alkylamino butanones申请人:GEIGY AG J R公开号:US02344814A1公开(公告)日:1944-03-21
552,454. Aryloxy-alkylamino butanones. GEIGY AKT.-GES., J. R. Oct. 7, 1941, No. 12937. Convention date, Oct. 8, 1940. [Class 2 (iii)] Aryloxy-alkylamino butanones are prepared by first condensing a monovalent phenol or a derivative of a polyvalent phenol containing only one hydroxyl group with a monohalogen acetone and then treating the aryloxy acetone so produced with a hydroxymethylamine, In place of the hydroxymethylamine there may be employed the reaction mixture obtained from formaldehyde and a primary or a secondary amine. The butanones thus obtained may be reduced to the corresponding butanols. In examples (1) Monochloracetone is condensed with o-cresol in the presence of sodium hydroxide and the o-cresoxyacetone so produced treated with the crude condensation product obtained by reacting piperidine with formaldehyde. The resulting o-cresoxybutanonyl-piperidine 1- (2<;SP>;1<;/SP>; - methylphenoxy - 1') - 4 - piperidyl - butanone-2 is purified by distillation. (2) A benzene solution of o-cresoxyacetone is treated with diethylamine to give 1-(2<;SP>;1<;/SP>;-methyl-phenoxy- 1<;SP>;1<;/SP>;) - 4 - diethylaminobutanone - 2. Similarly using #-naphthol in place of cresol the corresponding #-naphthoxy compound is obtained. (3) Guaiacoxy-acetone from guaiacol and monochloracetone is reacted with the crude condensation product of formaldehyde and piperidine to give 1-(2<;SP>;1<;/SP>;-methoxyphenoxy-1<;SP>;1<;/SP>;)-4- piperidyl-butanone 2. (4) The condensation product of morpholine and formaldehyde is reacted with methoxyphenoxy-acetone to give 1 - (21 - methoxyphenoxy - 1<;SP>;1<;/SP>;) - 4 - morpholinyl - butanone-2. (5) o-Benzyloxyphenoxy acetone prepared by treating pyro-catechine mono-benzyl ether with monochloracetone in the presence of sodium hydroxide is added to a cold mixture of piperidine, formaldehyde, benzene and sodium chloride to give 1-(21-benzyloxyphenoxy)-4-piperidyl-butanone-2. The free amino phenol may be obtained by acid hydrolysis. (6) Benzyloxyphenoxy acetone is added to the reaction mixture of methylcyclohexylamine and formaldehyde and worked up as in example (5) to give 1-(2'-benzyloxyphenoxy) - 4 - (methylcyclohexylamino) - butanone-2. The free amino-phenol is obtained by acid hydrolysis. (7) o-Benzyloxyphenoxyacetone prepared as in example (5) are added to a benzene solution of morpholine, formaldehyde and sodium chloride to give 1-(21- benzyloxyphenoxy-1<;SP>;1<;/SP>;)-4-morpholinyl-butanone- 2. The amino ketone is reduced by sodium in alcohol to form 1-(2<;SP>;1<;/SP>;-hydroxyphenoxy- 1<;SP>;1<;/SP>;)-4-morpholinyl-butanone-2. (8) Phenoxyacetone in benzene solution is added to morpholine and formaldehyde to form 1- phenoxy-4-morpholinyl-butanone-2. The corresponding 1 - phenoxy - 4 - morpholinyl - butanol-2 is obtained by reduction of the ketone by sodium in alcohol.
552,454. 乙氧基-烷基氨基丁酮。GEIGY AKT.-GES.,J.R. 1941年10月7日,第12937号专利。公约日期,1940年10月8日。[第2类(iii)] 乙氧基-烷基氨基丁酮首先通过将一价酚或仅含有一个羟基的多价酚衍生物与一卤代丙酮缩合,然后用羟甲基胺处理生成的乙氧基丙酮来制备。在羟甲基胺的位置上,也可以使用由甲醛和一级或二级胺得到的反应混合物。所得的丁酮可以还原为相应的丁醇。在示例中(1)单氯代丙酮与邻甲酚在氢氧化钠存在下缩合,然后用通过对哌啶与甲醛反应得到的粗缩合产物处理所产生的邻甲氧基丙酮。通过蒸馏纯化得到的邻甲氧基丁酰基哌啶1-(2'-甲基苯氧-1')-4-哌啶基-丁酮-2。 (2)邻甲氧基丙酮的苯溶液与二乙胺反应生成1-(2'-甲基苯氧-1')-4-二乙基氨基丁酮-2。类似地,使用α-萘酚代替甲酚可得到相应的α-萘氧化合物。 (3)从愈创木酚和单氯代丙酮得到的愈创氧基丙酮与甲醛和哌啶的粗缩合产物反应,得到1-(2'-甲氧苯氧-1')-4-哌啶基-丁酮2。 (4)吗啉和甲醛的缩合产物与甲氧苯氧基丙酮反应,得到1-(2'-甲氧苯氧-1')-4-吗啉基-丁酮-2。 (5)通过将苯并二苯基醚与单氯代丙酮在氢氧化钠存在下反应制备的邻苯氧基丙酮添加到哌啶、甲醛、苯和氯化钠的冷混合物中,得到1-(2'-苯氧苯氧)-4-哌啶基-丁酮-2。通过酸水解可以得到游离氨基酚。 (6)苯氧基丙酮添加到甲基环己胺和甲醛的反应混合物中,并按照示例(5)的方法处理,得到1-(2'-苯氧苯氧)-4-(甲基环己基氨基)-丁酮-2。通过酸水解可以得到游离氨基酚。 (7)如示例(5)中制备的邻苯氧基丙酮添加到吗啉、甲醛和氯化钠的苯溶液中,得到1-(2'-苯氧苯氧-1')-4-吗啉基-丁酮-2。氨基酮通过酒精中的钠还原形成1-(2'-羟基苯氧-1')-4-吗啉基-丁酮-2。 (8)苯氧基丙酮在苯中加入吗啉和甲醛,形成1-苯氧基-4-吗啉基-丁酮-2。通过酒精中的钠还原酮可以得到相应的1-苯氧基-4-吗啉基-丁醇-2。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
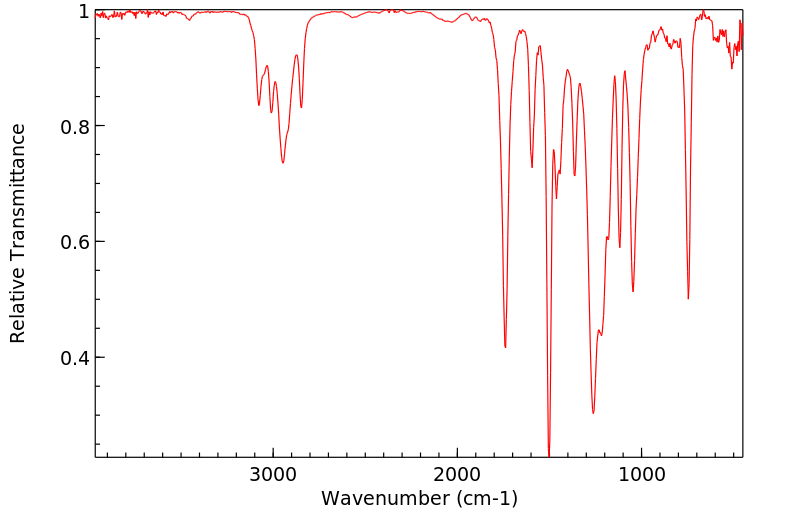
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息