S-(4-methoxybenzoyl)thiohydroxylamine | 35124-66-4
中文名称
——
中文别名
——
英文名称
S-(4-methoxybenzoyl)thiohydroxylamine
英文别名
S-Anisoylhydrosulfamin;S-(p-Anisoyl)hydrosulfamin;S-(p-Methoxybenzoyl)thiohydroxylamine;S-amino 4-methoxybenzenecarbothioate
CAS
35124-66-4
化学式
C8H9NO2S
mdl
——
分子量
183.231
InChiKey
IDUOPXWIQNWKEZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:352.6±44.0 °C(Predicted)
-
密度:1.254±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:77.6
-
氢给体数:1
-
氢受体数:4
上下游信息
反应信息
-
作为反应物:描述:S-(4-methoxybenzoyl)thiohydroxylamine 在 吡啶 、 高氯酸 、 乙酸酐 作用下, 以 二氯甲烷 为溶剂, 反应 19.0h, 生成 2,5-bis(4-methoxyphenyl)-1,3,4-oxathiazol-1-ium;perchlorate参考文献:名称:第一个草根zo鎓阳离子-1,3,4-草根zo鎓盐的简单合成摘要:1,3,4-氧杂噻唑鎓盐(,)是六种可能的芳族草硫唑鎓阳离子的首批代表,可以很容易地通过将-酰基硫羟胺()酰化,然后用强酸和苯甲酰环化来制备。酰基酐。与类似的二噻唑鎓盐相比,正电荷似乎离域的程度更小。DOI:10.1016/s0040-4039(00)97998-x
-
作为产物:描述:4-methoxythiobenzoic acid 在 potassium hydroxide 、 hydroxylamine-O-sulfonic acid 作用下, 以 水 为溶剂, 反应 0.08h, 生成 S-(4-methoxybenzoyl)thiohydroxylamine参考文献:名称:Design, Synthesis, and Cardioprotective Effects of N-Mercapto-Based Hydrogen Sulfide Donors摘要:Hydrogen sulfide (H2S) is a signaling molecule which plays regulatory roles in many physiological and/or pathological processes. Therefore, regulation of H2S levels could have great potential therapeutic value. In this work, we report the design, synthesis, and evaluation of a class of N-mercapto (N-SH)-based H2S donors. Thirty-three donors were synthesized and tested. Our results indicated that controllable H2S release from these donors could be achieved upon structural modifications. Selected donors (NSHD-1, NSHD-2, and NSHD-6) were tested in cellular models of oxidative damage and showed significant cytoprotective effects. Moreover, NSHD-1 and NSHD-2 were also found to exhibit potent protective effects in a murine model of myocardial ischemia reperfusion (MI/R) injury.DOI:10.1021/acs.jmedchem.5b01033
文献信息
-
<i>S</i>-Aroylthiooximes: A Facile Route to Hydrogen Sulfide Releasing Compounds with Structure-Dependent Release Kinetics作者:Jeffrey C. Foster、Chadwick R. Powell、Scott C. Radzinski、John B. MatsonDOI:10.1021/ol500385a日期:2014.3.21We report the facile preparation of a family of S-aroylthiooxime (SATO) H2S donors, which are synthesized via a click reaction analogous to oxime formation between S-aroylthiohydroxylamines (SATHAs) and aldehydes or ketones. Analysis of cysteine-triggered H2S release revealed structure-dependent release kinetics with half-lives from 8–82 min by substitution of the SATHA ring. The pseudo-first-order
-
Supramolecular Tuning of H<sub>2</sub>S Release from Aromatic Peptide Amphiphile Gels: Effect of Core Unit Substituents作者:Yun Qian、Kuljeet Kaur、Jeffrey C. Foster、John B. MatsonDOI:10.1021/acs.biomac.8b01732日期:2019.2.11substituents on the SATO group affected H2S release rates predictably in line with electronic effects (Hammett σ values) according to a linear free energy relationship. Above the CAC, circular dichroism, infrared, and fluorescence spectroscopies demonstrated that substituents influenced the self-assembled structures by affecting hydrogen bonding (β-sheet formation) and π-π stacking. At these concentrations, electronicH2S是一种具有多种生理作用的气体递质,但是其反应性和在生物介质中的半衰期短,使其难以控制地传递。对于气体的生物应用,水凝胶具有传递 的潜力,与其他供体系统相比具有许多优势,包括局部传递,控制释放速率,生物降解和可变的机械性能。在这项研究中,我们设计和评估了可控的 输送的基于肽的 释放水凝胶。这些水凝胶是由短的,自组装的芳香族肽两亲物(APA)制备的,在其N端被S-芳基硫代肟(SATO)官能化,后者会响应硫醇触发而释放 。研究了APA的溶液形式和凝胶形式,并通过添加CAChref=https://www.molaid.com/MS_35596 target="_blank">CaCl2引发了凝胶作用。APA的SATO组分上包含各种取代基,以评估它们对溶液相和凝胶相中自组装形态和 释放速率的影响。透射电子显微镜(TEM)图像证实,所有释放 的APA均自组装成纳米纤维,高于临界聚集浓度(CAC)约〜0.5 mg / mL。在CAC以下,SATO基团上的取代基根据线性自由能关系,可
-
YUNG, TOMMY W. K.;SAMMES, MICHAEL P., TETRAHEDRON LETT., 31,(1990) N1, C. 5935-5938作者:YUNG, TOMMY W. K.、SAMMES, MICHAEL P.DOI:——日期:——
表征谱图
-
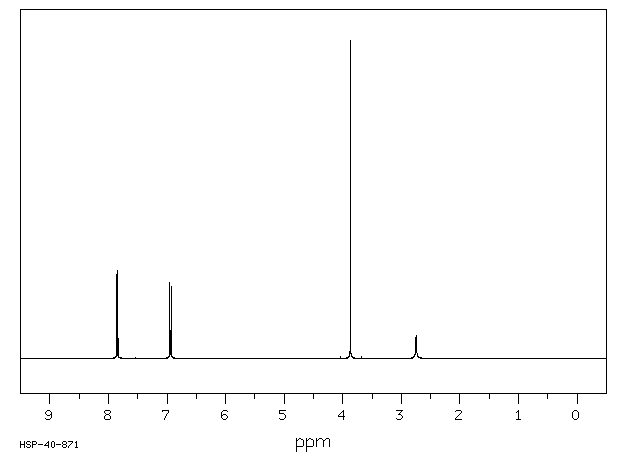
氢谱1HNMR
-
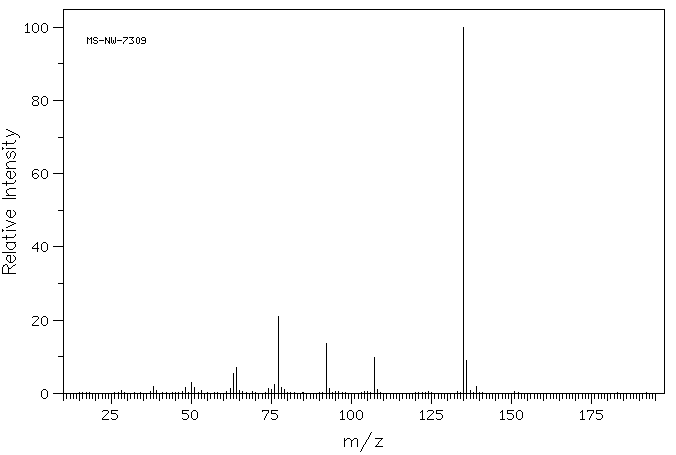
质谱MS
-
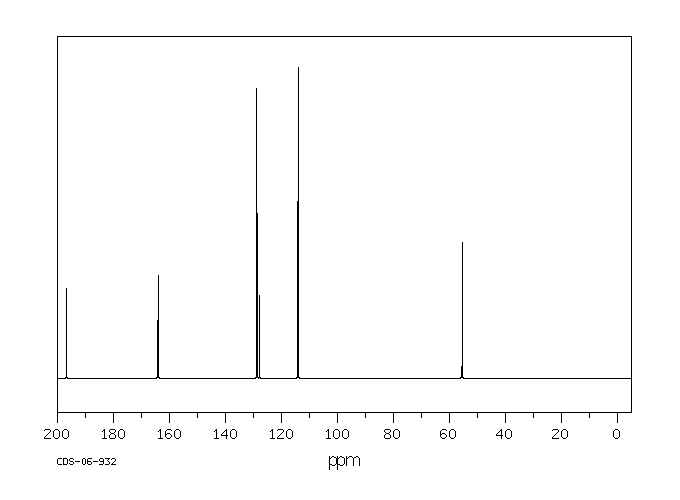
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫