(Z)-(S)-12-hydroxyoctadec-9-enoic acid methyl ester | 84799-82-6
中文名称
——
中文别名
——
英文名称
(Z)-(S)-12-hydroxyoctadec-9-enoic acid methyl ester
英文别名
methyl (S)-ricinoleate;methyl ricinoleate;Ricinolsaeure-methylester;methyl (Z,12S)-12-hydroxyoctadec-9-enoate
CAS
84799-82-6
化学式
C19H36O3
mdl
——
分子量
312.493
InChiKey
XKGDWZQXVZSXAO-GFBZKKKVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:22
-
可旋转键数:16
-
环数:0.0
-
sp3杂化的碳原子比例:0.84
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2918199090
SDS
上下游信息
反应信息
-
作为反应物:描述:(Z)-(S)-12-hydroxyoctadec-9-enoic acid methyl ester 在 甲醇 、 potassium hydroxide 、 盐酸 作用下, 以 水 为溶剂, 反应 2.0h, 以1.98 g的产率得到(Z)-(S)-12-hydroxyoctadec-9-enoic acid参考文献:名称:Synthesis of (S)-ricinoleic acid and its methyl ester with the participation of ionic liquid摘要:(R)-Ricinoleic acid methyl ester obtained from commercial castor oil was transformed in a three-step procedure into its S-enantiomer in overall 36% yield using ionic liquid (1-butyl-3-methylimidazolium acetate) in the key step process. The developed procedure provides easy access to (S)-ricinoleic acid and its methyl ester of over 95% enantiomeric excess. Optical rotations of the newly obtained compounds as well as their chromatographic and spectral characteristics are provided and discussed in the context of enantiopurity both of the substrate material and the final products. (C) 2014 Elsevier Ireland Ltd. All rights reserved.DOI:10.1016/j.chemphyslip.2014.06.005
-
作为产物:参考文献:名称:Synthesis of (S)-ricinoleic acid and its methyl ester with the participation of ionic liquid摘要:(R)-Ricinoleic acid methyl ester obtained from commercial castor oil was transformed in a three-step procedure into its S-enantiomer in overall 36% yield using ionic liquid (1-butyl-3-methylimidazolium acetate) in the key step process. The developed procedure provides easy access to (S)-ricinoleic acid and its methyl ester of over 95% enantiomeric excess. Optical rotations of the newly obtained compounds as well as their chromatographic and spectral characteristics are provided and discussed in the context of enantiopurity both of the substrate material and the final products. (C) 2014 Elsevier Ireland Ltd. All rights reserved.DOI:10.1016/j.chemphyslip.2014.06.005
文献信息
-
Antimicrobial Potential of Chiral Amide Derivatives of Ricinoleic and 3‐Hydroxynonanoic Acid作者:Sylwia Matysiak、Julia Zabielska、Józef Kula、Alina Kunicka‐StyczyńskaDOI:10.1002/aocs.12292日期:2020.1obtained compounds showed antimold potential; however, the tested species of molds were more susceptible to derivatives of 3‐hydroxynonanoic acid than to amides obtained from ricinoleic acid (RA). Interestingly, hydroxamic acids derived from RA exhibited the best activity against Candida albicans and Candida tropicalis. On the other hand, hydroxamic acids derived from 3‐hydroxynonanoic acid showed the最近已经观察到脂肪酸酰胺在药物化学中的作用越来越大。因此,使用简单快速的方法,可以得到一系列蓖麻油酸和3-羟基壬酸的手性酰胺衍生物(24种化合物),产率为31–95%。然后,评估了它们对代表革兰氏阳性和革兰氏阴性细菌,酵母菌和霉菌的13种微生物的抗菌活性。所获得的化合物显示出抗霉变的潜力。但是,测试过的霉菌种类对3-羟基壬酸衍生物的敏感性要高于从蓖麻油酸(RA)获得的酰胺。有趣的是,源自RA的异羟肟酸对白色念珠菌和热带念珠菌表现出最好的活性。。另一方面,衍生自3-羟基壬酸的异羟肟酸对其余测试微生物,尤其是对雪松假单胞菌具有最佳的抗菌潜力。所获得的衍生物可以被认为是具有潜在药理学意义的化合物,由于日益增加的微生物抗性问题,这是重要的。
-
Chiral amide derivatives of ricinoleic acid and 3-hydroxynonanoic acid synthesis and cytotoxic activity作者:Sylwia Matysiak、Józef Kula、Alina BłaszczykDOI:10.1007/s00044-019-02348-y日期:2019.7series of chiral ricinoleic and 3-hydroxynonanoic acid derivatives were synthesized in this study using various chemical and biochemical procedures. An effective method for preparation of methyl esters of 3-hydroxynonanoic acid from castor oil or methyl ricinoleate by ozonolysis and oxidation was developed. Simple, fast, and efficient procedures were applied to obtain different primary and secondary
-
Synthesis of (<i>R</i>)- and (<i>S</i>)-Ricinoleic Acid Amides and Evaluation of Their Antimicrobial Activity作者:Sylwia Matysiak、Julia Zabielska、Józef Kula、Alina Kunicka-StyczyńskaDOI:10.1002/aocs.12013日期:2018.1fatty acid amides derived from (R)‐ and (S)‐ricinoleic acid and 4 cyclic and acyclic amines were synthesized in a proecological solvent‐free process with yields ranging from 43 to 88%. All S‐configured compounds and both enantiomers of amide with 2‐amino‐2‐methyl‐1‐propanol were obtained and studied in terms of biological activity for the first time. The evaluation of antimicrobial activity of (R)‐ and在无保护性溶剂的过程中合成了一系列衍生自(R)和(S)蓖麻油酸的脂肪酸酰胺和4个环胺和无环胺,收率范围为43%至88%。首次获得了所有S构型的化合物以及酰胺与2-氨基-2-甲基-1-丙醇的两种对映异构体,并首次就生物学活性进行了研究。(R)和(S)-蓖麻油酸衍生物对代表革兰氏阴性和革兰氏阳性细菌,酵母和霉菌的13种不同微生物的抗菌活性评估显示出对革兰氏阳性细菌特别是黄球菌和微球菌的显着抑制活性。枯草芽孢杆菌,并针对选定的霉菌。乙醇胺和吡咯烷衍生的酰胺显示出最有希望的抗菌和抗霉菌潜力。蓖麻油酸和吡咯烷的衍生物对选择的两种霉菌,巴西曲霉和扩张青霉的活性最高。而且,R-构型的类似物对枯草芽孢杆菌最有效。蓖麻油酸与乙醇胺的酰胺对金黄色葡萄球菌显示出显着的潜力,这使它们与该细菌具有很高抗性的其余测试衍生物区分开来。
-
Synthesis and cytotoxicity of (<i>R</i>)- and (<i>S</i>)-ricinoleic acid amides and their acetates作者:Sylwia Matysiak、Agnieszka Chmiel、Janusz Skolimowski、Jozef Kula、Beata Pasternak、Alina BlaszczykDOI:10.1002/chir.22733日期:2017.10An environment‐friendly, free of solvent, process for the synthesis of (R)‐ and (S)‐ricinoleic acid amides has been developed. Starting from methyl ricinoleates and pyrrolidine or ethanolamine, the corresponding amides were obtained with yields ranging from 83–88%. Among 12 synthesized derivatives of ricinoleic acid, including the starting methyl esters, amides, and their acetates, nine compounds were
-
BoC<sub>2</sub>O Mediated Macrolactonisation: Syntheses of R-(+)-Ricinoleic Acid Lactone and (±)-12-OH-Stearic Acid Lactone作者:M. NagarajanDOI:10.1080/00397919908086253日期:1999.7An efficient chemoenzymatic synthesis of R-(+)-ricinoleic acid lactone and (+/-)-12-OH-stearic acid lactone using Boc(2)O mediated macrolactonisation is reported.
表征谱图
-
氢谱1HNMR
-
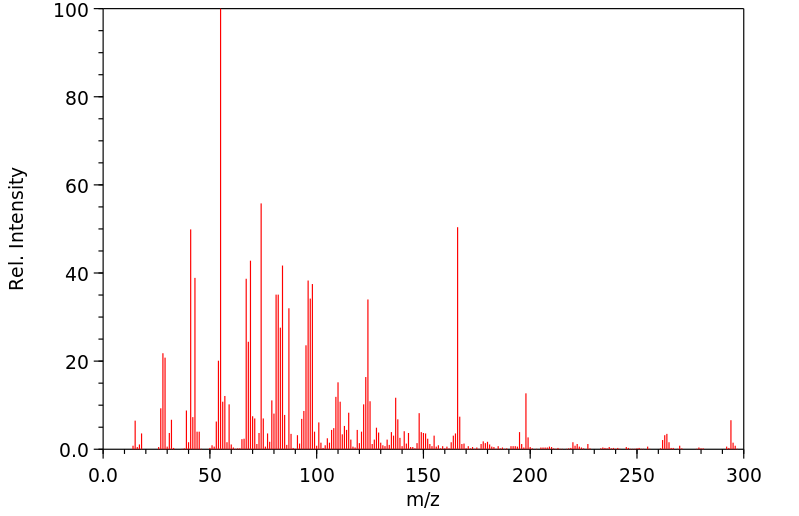
质谱MS
-
碳谱13CNMR
-
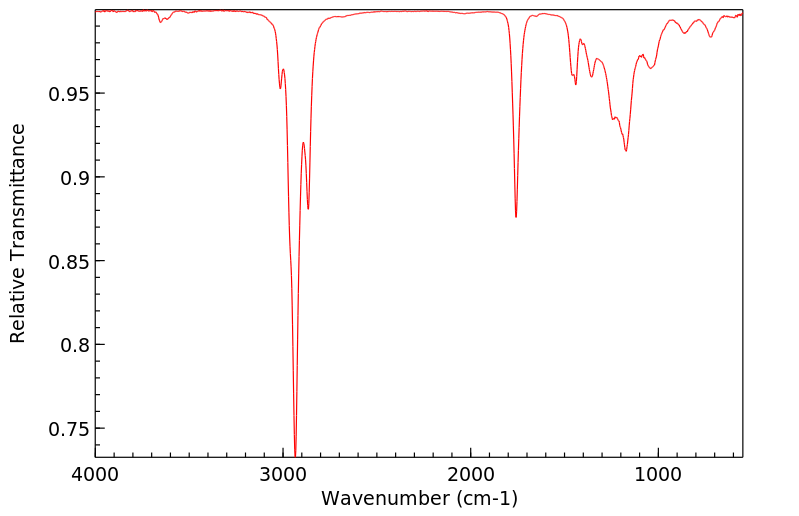
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯