N-((1E)-3-(phenylimino)prop-1-enyl)benzenamine hydrochloride | 873293-47-1
中文名称
——
中文别名
——
英文名称
N-((1E)-3-(phenylimino)prop-1-enyl)benzenamine hydrochloride
英文别名
N-((1E)-3-(phenylimino)prop-1-enyl)benzenamine;3-Anilinoacrolein anil;N-[(E)-3-phenyliminoprop-1-enyl]aniline
CAS
873293-47-1
化学式
C15H14N2
mdl
——
分子量
222.29
InChiKey
ZFYNTCNECVPHDZ-RMPAWCBFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:361.9±38.0 °C(Predicted)
-
密度:0.98±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:17
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:24.4
-
氢给体数:1
-
氢受体数:2
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-anilino-propenal 25299-39-2 C9H9NO 147.177
反应信息
-
作为反应物:描述:N-((1E)-3-(phenylimino)prop-1-enyl)benzenamine hydrochloride 在 sodium azide 、 L-ascorbic acid sodium salt 、 copper(II) sulfate 作用下, 以 四氢呋喃 、 水 、 乙酸酐 、 N,N-二甲基甲酰胺 、 叔丁醇 为溶剂, 反应 21.5h, 生成参考文献:名称:[EN] NOVEL PROBES AND TARGETING COMOUNDS FOR MITOCHONDRIA
[FR] NOUVELLES SONDES ET COMPOSÉS DE CIBLAGE DES MITOCHONDRIES摘要:公开号:WO2014063033A3 -
作为产物:描述:1,1,3,3-四甲氧基丙烷 、 苯胺 在 盐酸 作用下, 以 水 为溶剂, 以49%的产率得到N-((1E)-3-(phenylimino)prop-1-enyl)benzenamine hydrochloride参考文献:名称:[EN] NOVEL PROBES AND TARGETING COMOUNDS FOR MITOCHONDRIA
[FR] NOUVELLES SONDES ET COMPOSÉS DE CIBLAGE DES MITOCHONDRIES摘要:公开号:WO2014063033A3
文献信息
-
Hydrolysis of malonaldehyde dianil and .BETA.-arylaminoacrolein derivatives.作者:SHINZO TAMURA、MACHIKO ONO、KAZUMI FURUYAMADOI:10.1248/cpb.28.2356日期:——The reversible hydrolysis of β-arylaminoacrolein to form arylamine and malonaldehyde was studied kinetically. The catalytic coefficient of hydronium ions (kH+) and dissociation constant of the conjugate acid of β-arylaminoacrolein (KBH+) were evaluated. Hammett plots for kH+ and for KBH+ were linear. The values of log kH+ and pKBH+ were expressed by the equations log kH+=1.38σ-2.81 and pKBH+=-1.20σ+0.90, respectively. The reversible hydrolysis of malonaldehyde dianil to form β-arylaminoacrolein and arylamine was examined in relation to that of β-arylaminoacrolein. The preparation of β-arylaminoacrolein by hydrolysis of malonaldehyde dianil was achieved under weakly acidic conditions.
-
Efficient reverse click labeling of azide oligonucleotides with multiple alkynyl Cy-Dyes applied to the synthesis of HyBeacon probes for genetic analysis作者:Marta Gerowska、Lucy Hall、James Richardson、Montserrat Shelbourne、Tom BrownDOI:10.1016/j.tet.2011.11.041日期:2012.1A convenient method of oligonucleotide labeling using click chemistry has been developed. A 2'-mesyloxyethyl ribothymidine phosphoramidite monomer was incorporated into DNA at several loci during solid phase oligonucleotide synthesis and converted to 2'-azidoethyl ribothymidine in high yield on the synthesis resin. The resultant azide oligonucleotides were doubly and triply labeled with alkynemodified cyanine dyes and their biophysical properties were studied. The influence of the dye structures and method of labeling on the fluorescence properties of the DNA probes is discussed and compared with a standard labeling method using active esters of Cy-Dyes. (C) 2011 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
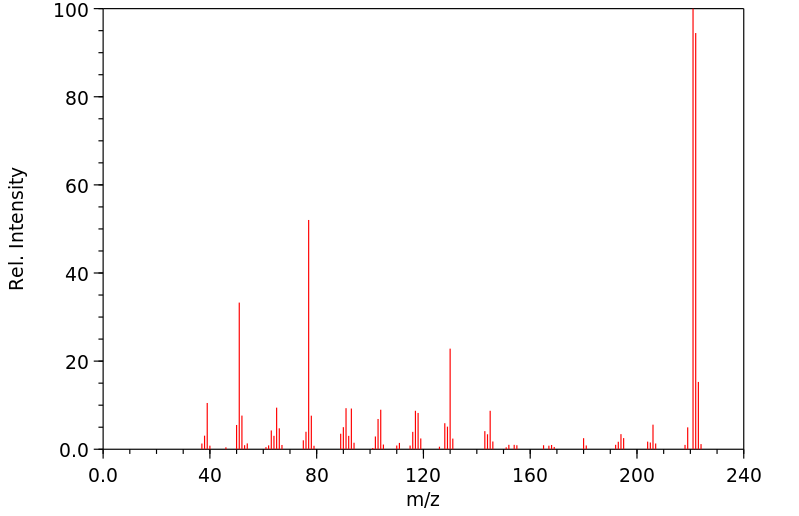
质谱MS
-
碳谱13CNMR
-
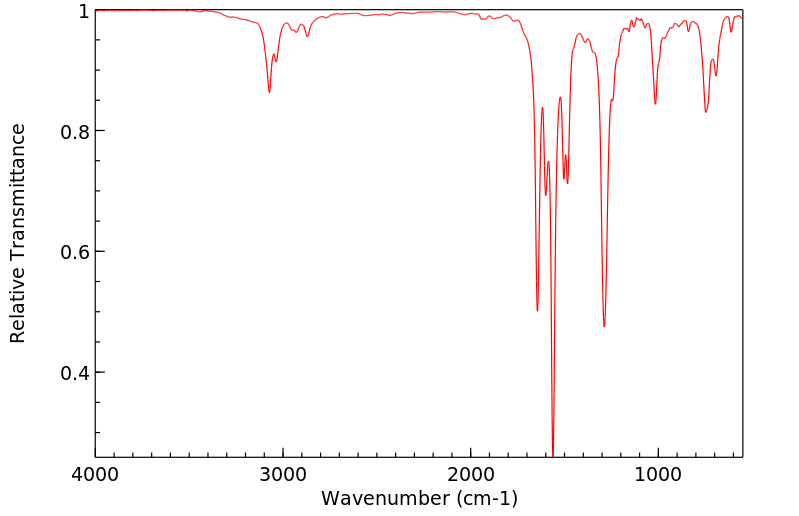
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫