联苯-3,4-二醇 | 92-05-7
中文名称
联苯-3,4-二醇
中文别名
3.4-联苯二酚
英文名称
3,4-dihydroxybiphenyl
英文别名
4-phenyl pyrocatechol;3',4'-dihydroxy-1,1'-biphenyl;[1,1'-biphenyl]-3,4-diol;4-Phenylpyrocatechol;biphenyl-3,4-diol;4-phenylbenzene-1,2-diol
CAS
92-05-7
化学式
C12H10O2
mdl
MFCD00041746
分子量
186.21
InChiKey
QDNPCYCBQFHNJC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:139.35°C
-
沸点:280.69°C (rough estimate)
-
密度:1.1032 (rough estimate)
-
溶解度:0.01 M
-
保留指数:1918
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
SDS
| Name: | 4-Phenylpyrocatechol 98% (Titr.) Material Safety Data Sheet |
| Synonym: | 1,1'-Biphenyl-3,4-Diol |
| CAS: | 92-05-7 |
Synonym:1,1'-Biphenyl-3,4-Diol
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 92-05-7 | 1,1'-Biphenyl-3,4-Diol | 98 | 202-121-6 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 92-05-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white to off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 138 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C12H10O2
Molecular Weight: 186.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 92-05-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,1'-Biphenyl-3,4-Diol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 92-05-7: No information available.
Canada
CAS# 92-05-7 is listed on Canada's NDSL List.
CAS# 92-05-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 92-05-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 间羟基联苯 3-phenylphenol 580-51-8 C12H10O 170.211 3,4-DIMETHOXYBIPHENYL锛圵S201555锛,WUXIAPPTEC" 3,4-dimethoxy-1,1'-biphenyl 17423-55-1 C14H14O2 214.264 对羟基联苯 4-Phenylphenol 92-69-3 C12H10O 170.211 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-hydroxy-4-methoxybiphenyl 37055-80-4 C13H12O2 200.237
反应信息
-
作为反应物:描述:参考文献:名称:邻喹啉酮和苯酚的催化剂控制的需氧耦合用于芳醚的合成摘要:邻-喹诺酮是未充分利用的六碳原子构建基块。我们在本文中描述了一种控制其与铜的反应性的方法,该方法引起了与苯酚的催化好氧交叉偶联。在温和条件下,可在宽范围的底物范围内以高收率生成所得芳基醚。该方法代表了一个独特的例子,其中过渡金属催化了邻醌的共价修饰,为它们在合成中的利用创造了新的机会。DOI:10.1002/anie.201606359
-
作为产物:描述:参考文献:名称:邻喹啉酮和苯酚的催化剂控制的需氧耦合用于芳醚的合成摘要:邻-喹诺酮是未充分利用的六碳原子构建基块。我们在本文中描述了一种控制其与铜的反应性的方法,该方法引起了与苯酚的催化好氧交叉偶联。在温和条件下,可在宽范围的底物范围内以高收率生成所得芳基醚。该方法代表了一个独特的例子,其中过渡金属催化了邻醌的共价修饰,为它们在合成中的利用创造了新的机会。DOI:10.1002/anie.201606359
文献信息
-
Synthesis of α-oxygenated ketones and substituted catechols via the rearrangement of N-enoxy- and N-aryloxyphthalimides作者:Michelle A. Kroc、Aditi Patil、Anthony Carlos、Josiah Ballantine、Stephanie Aguilar、Dong-Liang Mo、Heng-Yen Wang、Daniel S. Mueller、Donald J. Wink、Laura L. AndersonDOI:10.1016/j.tet.2017.01.061日期:2017.7synthesis of α-oxygenated carbonyl compounds and catechols is the treatment of a carbonyl compound or a phenol with an electrophilic oxygen source. As an alternative approach to these important structures, formal [3,3]-rearrangements of N-enoxyphthalimides, N-enoxyisoindolinones, and N-aryloxyphthalimides have been explored. When used in combination with an initial Chan-Lam coupling, these transformations
-
Altering 2-Hydroxybiphenyl 3-Monooxygenase Regioselectivity by Protein Engineering for the Production of a New Antioxidant作者:Almog Bregman-Cohen、Batel Deri、Shiran Maimon、Yael Pazy、Ayelet FishmanDOI:10.1002/cbic.201700648日期:2018.3.16New regiospecificity→new antioxidant: One single mutation, M321A, changed 2‐hydroxybiphenyl 3‐monooxygenase (HbpA) regioselectivity from oxidizing 2‐substituted phenols to 3‐substituted phenols. The crystal structure provided an explanation for the new activity. The antioxidant produced, 3,4‐dihydroxybiphenyl, had similar ferric reducing capacity to the well‐studied piceatannol.
-
Photoinduced reactions—XXXV作者:K. Omura、T. MatsuuraDOI:10.1016/0040-4020(70)85026-8日期:——compounds as the main product. Relative reactivity of representative substituted phenols and the effect of the phenols on the decomposition rate of hydrogen peroxide were examined by a competition reaction with p-cresol. The apparent reactivity of phenols was found to decrease in the following order; p-Ph >p-Ac, p-Me, p-Cl > p-COOH, o-NO2, p-CN, p-t-Bu > m-COOH, p-NO2 > 2,4-(COOMe)2 > 2,4(NO2)2. The
-
Conversion of Simple Cyclohexanones into Catechols作者:Yu-Feng Liang、Xinyao Li、Xiaoyang Wang、Miancheng Zou、Conghui Tang、Yujie Liang、Song Song、Ning JiaoDOI:10.1021/jacs.6b07269日期:2016.9.21A novel I2-catalyzed direct conversion of cyclohexanones to substituted catechols under mild and simple conditions has been described. This novel transformation is remarkable with the multiple oxygenation and dehydrogenative aromatization processes enabled just by using DMSO as the solvent, oxidant, and oxygen source. This metal-free and simple system demonstrates a versatile protocol for the synthesis
-
Melanin concentrating hormone antagonists申请人:Hu Eric Xiufeng公开号:US20050075324A1公开(公告)日:2005-04-07The present invention relates to compounds capable of serving as moderators of human and mammalian appetite and as such provides a means for reducing body mass. The compounds of the present invention are selective against melanin concentrating hormone and do not have the pernicious side effects resulting from compounds which interact with other appetite related brain receptors.本发明涉及能够作为人类和哺乳动物食欲调节剂的化合物,从而提供了一种减少体重的手段。本发明的化合物对黑色素浓缩激素具有选择性,并且不具有与与其他食欲相关的脑受体相互作用的化合物导致的有害副作用。
表征谱图
-
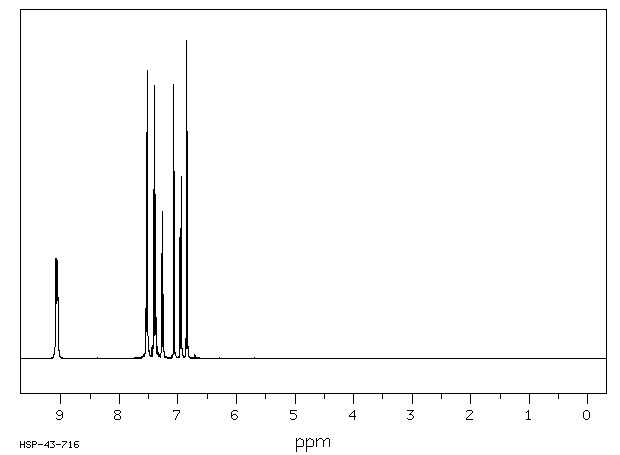
氢谱1HNMR
-
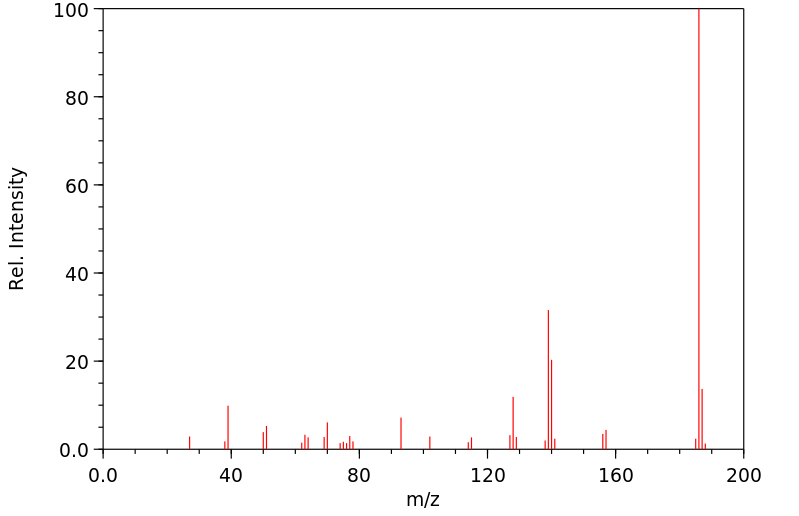
质谱MS
-
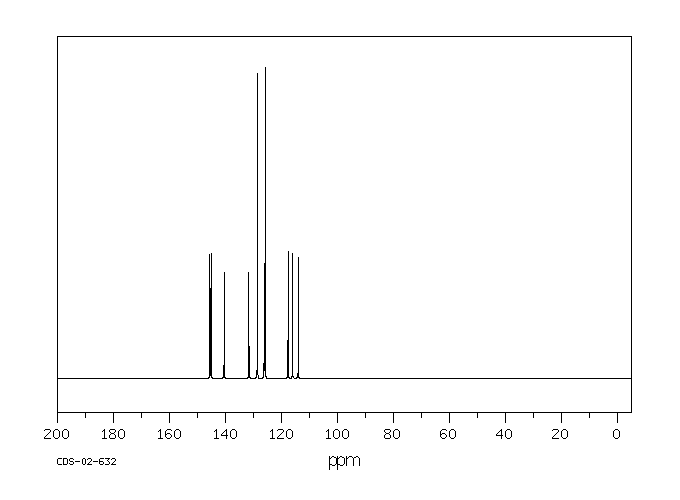
碳谱13CNMR
-
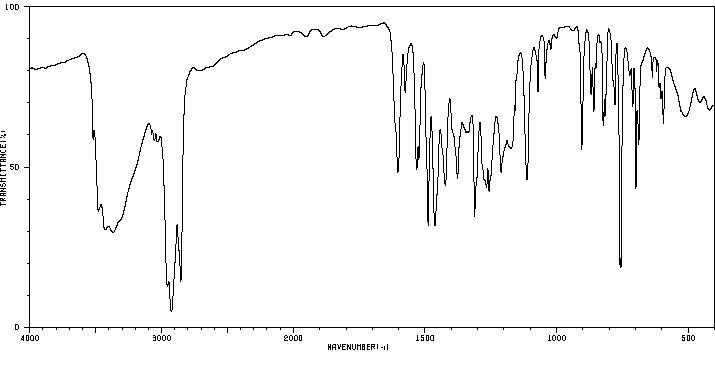
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫