N-(3-bromophenyl)-4-methyl-benzenesulfonamide | 7510-48-7
中文名称
——
中文别名
——
英文名称
N-(3-bromophenyl)-4-methyl-benzenesulfonamide
英文别名
N-(3-bromophenyl)-4-methylbenzenesulfonamide;N-(3-bromophenyl)-p-toluenesulphonamide;4-Toluolsulfon-N-(3-brom-phenyl)-amid
CAS
7510-48-7
化学式
C13H12BrNO2S
mdl
MFCD01016286
分子量
326.214
InChiKey
HBZNLTGGGCUOMY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:429.7±55.0 °C(Predicted)
-
密度:1.545±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:54.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H315,H319,H335
-
储存条件:存储条件:室温、密封、干燥
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Tokuyama, Hidetoshi; Sato, Masashi; Ueda, Toshihiro, Heterocycles, 2001, vol. 54, # 1, p. 105 - 108摘要:DOI:
-
作为产物:描述:对甲苯磺酰氯 在 三甲基氯硅烷 、 氯化镍二甲氧基乙烷 、 4,7-二苯基-1,10-菲罗啉 、 三乙胺 、 锌 作用下, 以 1,4-二氧六环 、 二氯甲烷 为溶剂, 反应 25.0h, 生成 N-(3-bromophenyl)-4-methyl-benzenesulfonamide参考文献:名称:N-酰基和 N-磺酰基苯并三唑与多种硝基化合物的镍催化还原交叉偶联:快速获得酰胺和磺酰胺摘要:在此,我们报道了一种方便获得的N-酰基苯并三唑与烷基、烯基和芳基硝基化合物的 Ni 催化还原转酰胺基反应,该反应以良好的收率和广泛的底物范围提供了各种酰胺。相同的催化反应条件也适用于N-磺酰基苯并三唑,它可以与硝基芳烃和硝基烷烃进行平滑的还原偶联,得到相应的磺酰胺。DOI:10.1021/acs.orglett.1c03535
文献信息
-
A Convenient and Benign Synthesis of Sulphonamides in PEG-400作者:Pranab Jyoti Das、Bhaskar SarmahDOI:10.14233/ajchem.2015.16853日期:——A simple and convenient method is reported for the synthesis of a series of sulphonamides in PEG-400 using potassium carbonate as the base. The reaction is carried out in a heterogeneous medium and consequently product recovery is simple. The desired products with a variety of aromatic amines could be synthesized in a short reaction time in good yield. The PEG could be recovered for reuse.
-
Synthesis, Characterization, and Reactivity of an Ethynyl Benziodoxolone (EBX)–Acetonitrile Complex作者:Masaharu Yudasaka、Daisuke Shimbo、Toshifumi Maruyama、Norihiro Tada、Akichika ItohDOI:10.1021/acs.orglett.9b00005日期:2019.2.15The synthesis of a crystalline ethynyl-1,2-benziodoxol-3(1H)-one (EBX)–acetonitrile complex is described. EBX has been widely used as an active species for a variety of reactions; however, its high instability has so far prevented its isolation. The EBX–acetonitrile is self-assembled into a double-layered honeycomb structure through weak hypervalent iodine secondary interactions and hydrogen bonding
-
Direct oxidative nitration of aromatic sulfonamides under mild conditions作者:Ying-Xiu Li、Lian-Hua Li、Yan-Fang Yang、Hui-Liang Hua、Xiao-Biao Yan、Lian-Biao Zhao、Jin-Bang Zhang、Fa-Jin Ji、Yong-Min LiangDOI:10.1039/c4cc03784g日期:——
This efficient reaction proceeds under very mild conditions with low cost nitration reagents and can be applied in gram-scale preparation.
这种高效反应在非常温和的条件下进行,使用成本低廉的硝化试剂,可用于克级制备。 -
Synthesis of <i>cis</i>-β-Amidevinyl Benziodoxolones from the Ethynyl Benziodoxolone–Chloroform Complex and Sulfonamides作者:Daisuke Shimbo、Atsushi Shibata、Masaharu Yudasaka、Toshifumi Maruyama、Norihiro Tada、Bunji Uno、Akichika ItohDOI:10.1021/acs.orglett.9b03990日期:2019.12.6The synthesis of cis-β-amidevinyl benziodoxolones from the ethynyl benziodoxolone–chloroform complex and sulfonamides is reported. Evidence indicates that highly reactive unsubstituted ethynyl benziodoxolone undergoes Michael addition of sulfonamides, including sterically demanding acyclic amino acid derivatives. The synthesis of selectively deuterated cis-β-amidevinyl benziodoxolones and investigation
-
Palladium-Catalyzed Intramolecular Oxidative Coupling Involving Double C(sp<sup>2</sup>)–H Bonds for the Synthesis of Annulated Biaryl Sultams作者:Joydev K. Laha、Krupal P. Jethava、Neetu DayalDOI:10.1021/jo5011334日期:2014.9.5The palladium-catalyzed intramolecular oxidative coupling described herein involves a double C(sp2)–H bond functionalization in sulfonanilides, providing a workable access to biaryl sultams annulated into a six-membered ring that are otherwise difficult to obtain by literature methods. The other synthetic applications of this protocol including the synthesis of biaryl sultams containing a seven-membered
表征谱图
-
氢谱1HNMR
-
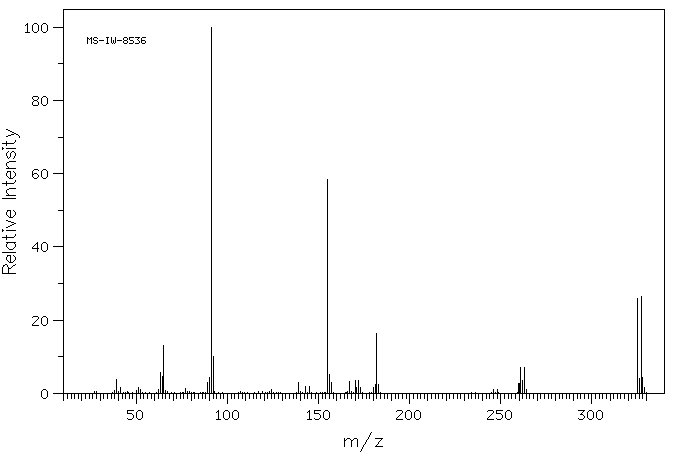
质谱MS
-
碳谱13CNMR
-
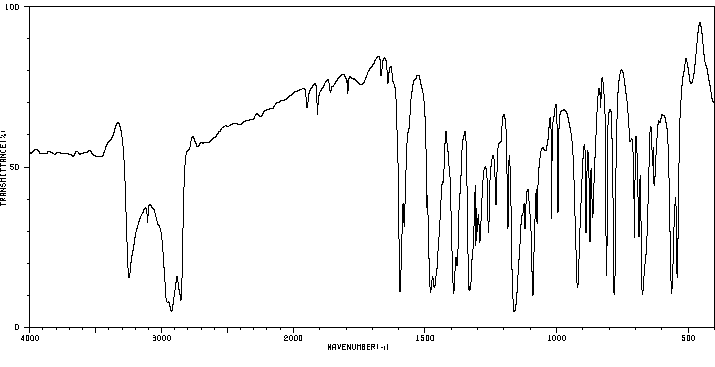
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫