5-(苯氧基甲基)-3-苯基-2-恶唑烷酮 | 1226-26-2
中文名称
5-(苯氧基甲基)-3-苯基-2-恶唑烷酮
中文别名
——
英文名称
5-phenoxymethyl-3-phenyloxazolidin-2-one
英文别名
3-phenyl-5-(phenoxymethyl)-1,3-oxazolidin-2-one;3-Phenyl-5-methylenephenoxyoxazolidin-2-one;5-(phenoxymethyl)-3-phenyl-oxazolidin-2-one;5-(phenoxymethyl)-3-phenyloxazolidin-2-one;3-phenyl-5-phenoxymethyl-2-oxazolidinone;3-phenyl-5-phenoxymethyloxazolidin-2-one;5-(phenoxymethyl)-3-phenyl-1,3-oxazolidin-2-one
CAS
1226-26-2
化学式
C16H15NO3
mdl
——
分子量
269.3
InChiKey
TVCILYMEQCWTGP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:20
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:38.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
上下游信息
反应信息
-
作为反应物:描述:劳森试剂 、 5-(苯氧基甲基)-3-苯基-2-恶唑烷酮 以 甲苯 为溶剂, 反应 8.0h, 以73%的产率得到(+/-)-5-(phenoxymethyl)-3-phenyl-1,3-oxazolidine-2-thione参考文献:名称:El-Barbary, A. A.; Lawesson, S. O., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1984, vol. 23, # 7, p. 655 - 657摘要:DOI:
-
作为产物:描述:methyl N-(2-hydroxy-3-phenoxypropyl)-N-phenylcarbamate 在 [(Me3Si)2N]3La(μ-Cl)Li(THF)3 作用下, 以 neat (no solvent) 为溶剂, 反应 12.0h, 生成 5-(苯氧基甲基)-3-苯基-2-恶唑烷酮参考文献:名称:稀土金属酰胺催化多组分合成恶唑烷酮摘要:已开发了稀土金属酰胺催化的环氧化物,胺和碳酸二甲酯的三组分反应,以合成恶唑烷酮。制备了47个3,5-二取代的恶唑烷酮实例,产率为13–97%。这是一种简单且最实用的方法,它使用易于获得的底物和催化剂,适用于各种芳族和脂族胺以及单取代的环氧化物。二取代的环氧化物的范围相当有限,这需要进一步研究。初步的机理研究揭示了通过β-氨基醇或酰胺中间体产生的两种可能的反应途径。DOI:10.1002/cctc.201900221
文献信息
-
Squaramide-Quaternary Ammonium Salt as an Effective Binary Organocatalytic System for Oxazolidinone Synthesis from Isocyanates and Epoxides作者:Ali Rostami、Amirhossein Ebrahimi、John Husband、Muhammad Usman Anwar、Rene Csuk、Ahmed Al-HarrasiDOI:10.1002/ejoc.202000153日期:2020.3.31organocatalyst for the atom‐economic conversion of a plethora of alkyl‐ and aryl‐substituted epoxides and isocyanates into oxazolidinones is described. A mechanism was proposed wherein the nucleophilic ring‐opening operation, and oxo‐ and carbamate‐anions stabilization occur cooperatively towards isocyanate fixation.
-
Selective Synthesis of 5‐Substituted <i>N</i> ‐Aryloxazolidinones by Cycloaddition Reaction of Epoxides with Arylcarbamates Catalyzed by the Ionic Liquid BmimOAc作者:Elnazeer H. M. Elageed、Bihua Chen、Binshen Wang、Yongya Zhang、Shi Wu、Xiuli Liu、Guohua GaoDOI:10.1002/ejoc.201600474日期:2016.75-substituted N-aryloxazolidinones in excellent yields. In addition, chiral 5-substituted oxazolidinones were synthesized by this procedure in good-to-excellent yields with enantiomeric excesses in excess of 99.9 % starting from chiral terminal epoxides. A possible reaction mechanism is discussed in accord with the results obtained by 1H NMR spectroscopy and DFT calculations, which indicate the cooperative activation
-
Transformation of Carbon Dioxide into Oxazolidinones and Cyclic Carbonates Catalyzed by Rare-Earth-Metal Phenolates作者:Bin Xu、Peng Wang、Min Lv、Dan Yuan、Yingming YaoDOI:10.1002/cctc.201600534日期:2016.8.8Rare‐earth‐metal complexes stabilized by amine‐bridged tri(phenolato) ligands were developed, and their activities in catalyzing transformations of CO2 were studied. A series of terminal epoxides and challenging disubstituted epoxides were converted into the respective cyclic carbonates in the presence of CO2 in yields of 58 to 96 %. In addition, these rare‐earth‐metal complexes also showed good activities
-
Microwave-Assisted Electrostatically Enhanced Phenol-Catalyzed Synthesis of Oxazolidinones作者:Ali Rostami、Amirhossein Ebrahimi、Nader Sakhaee、Farhad Golmohammadi、Ahmed Al-HarrasiDOI:10.1021/acs.joc.1c01686日期:2022.1.7one-component organocatalyst for the atom-economic transformation of epoxides to oxazolidinones under microwave irradiation. Integrating a positively charged center into phenols over a modular one-step preparation gives rise to a bifunctional system with improved acidity and activity, competent in rapid assembly of epoxides and isocyanates under microwave irradiation in a short reaction time (20–60 min)静电增强的苯酚被用作一种简单、可持续和有效的单组分有机催化剂,用于在微波照射下将环氧化物原子经济地转化为恶唑烷酮。通过模块化的一步制备将带正电荷的中心整合到酚类中,产生了一个双功能体系,具有改善的酸度和活性,能够在微波照射下在短反应时间(20-60 分钟)内快速组装环氧化物和异氰酸酯。仔细评估了各种带正电荷的苯酚和苯胺的功效,并检查了催化剂负载、温度和亲核试剂种类等几个因素对催化反应性的影响。在整洁的条件下,利用这一单组分催化平台,可在数分钟内从各种芳基和烷基取代的环氧化物和异氰酸酯制备 40 多个恶唑烷酮实例,实现了高达 96% 的收率和高选择性。进行了 DFT 计算以实现不同催化途径的反应势垒,以提供机理理解并证实了其中提出的同时环氧化物开环和异氰酸酯掺入的实验结果。
-
2-Oxazolidones from Glycidyl Ether Reactions with Acid Amides作者:Yoshio Iwakura、Shin-ichi IzawaDOI:10.1246/bcsj.39.2490日期:1966.11The reaction between acid amides and aryl glycidyl ethers was carried out using tertiary amine as the catalyst. 2-Oxazolidone derivatives were obtained by the reaction of trichloroacetanilide or trifluoroacetanilide with aryl glycidyl ether. Acyl migration occurred in the reaction of acetanilide with phenyl glycidyl ether.
表征谱图
-
氢谱1HNMR
-
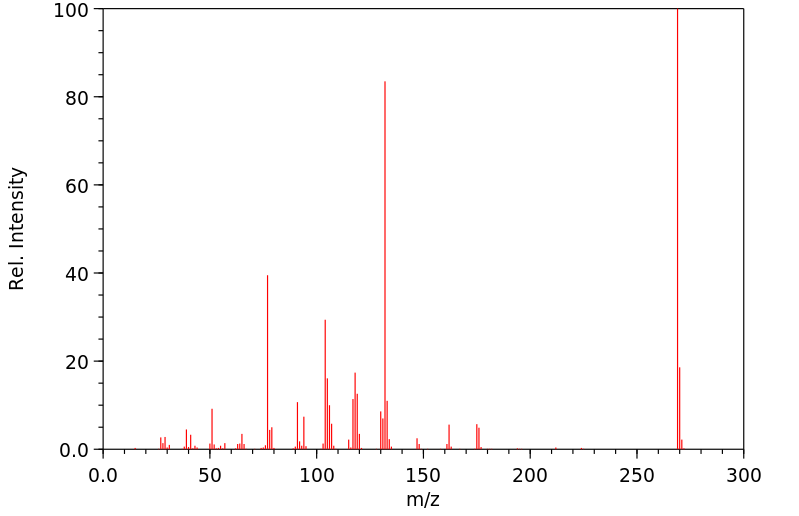
质谱MS
-
碳谱13CNMR
-
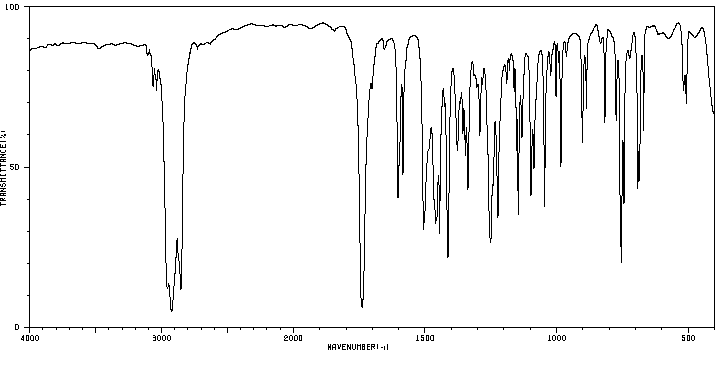
红外IR
-
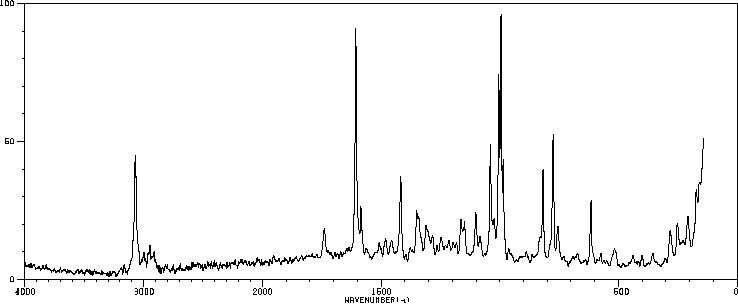
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯