S-(4-nitrophenyl) 4-methylbenzenesulfonothioate | 17046-97-8
中文名称
——
中文别名
——
英文名称
S-(4-nitrophenyl) 4-methylbenzenesulfonothioate
英文别名
S-4-nitrophenyl 4-methylbenzenesulfonothioate;S-4-nitrophenyl 4-toluenethiosulfonate;S-p-nitrophenyl p-toluenethiosulfonate;toluene-4-thiosulfonic acid S-(4-nitro-phenyl ester);Toluol-4-thiosulfonsaeure-S-(4-nitro-phenylester);p-nitrophenyl p-toluenethiosulfonate;1-Methyl-4-(4-nitrophenyl)sulfanylsulfonylbenzene
CAS
17046-97-8
化学式
C13H11NO4S2
mdl
——
分子量
309.367
InChiKey
XLTUAAUTJGKTSC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:20
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:114
-
氢给体数:0
-
氢受体数:5
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-甲基-4-[(4-硝基苯基)二硫烷基]苯 4-nitrophenyl p-tolyl disulfide 14756-51-5 C13H11NO2S2 277.368 4,4'-二硝基二苯二硫醚 di(p-nitrophenyl) disulfide 100-32-3 C12H8N2O4S2 308.339
反应信息
-
作为反应物:参考文献:名称:中性三原子组分与末端炔烃的 (3 + 2) 环加成反应中的铜和银催化摘要:将铜催化的叠氮化物-炔烃偶联 (CuAAC) 引入 1,3-偶极环加成反应对于其在合成化学中的普及以及在多个其他科学领域的应用至关重要。当造币金属乙炔化物中间体参与环化过程时观察到的反应速率提高极大地扩展了可以与末端炔发生(3+2)环加成的结构和条件范围。在此,我们报告了在性质和水平上相当的速率增强是由铜和银催化剂在末端炔烃与“中性”三原子组分(TAC)(特别是炔基硫醚)的分子内(3 + 2)环加成中引起的。通过对反应优化、实验和 DFT 机理研究的仔细观察,提出了一条涉及质子耦合环金属化关键步骤的途径。已开发的催化条件集使我们能够克服先前由“中性”TAC 的热促进 (3 + 2) 环加成所带来的几个范围限制,从而扩大了其合成和应用潜力。DOI:10.1021/jacs.3c06533
-
作为产物:参考文献:名称:Leandri; Tundo, Annali di Chimica, 1954, vol. 44, p. 63,69摘要:DOI:
文献信息
-
一种基于二氧化硫插入策略合成硫代磺酸酯 类化合物的方法
-
Sulfur containing compounds申请人:——公开号:US20030220524A1公开(公告)日:2003-11-27This invention is directed to novel and known stufur containing compounds and pharmaceutically acceptable salts thereof that have utility as antifungals and as antiproliferative agents against mammalian cells, in particular cancer cells and most particularly leukemia-derived cells. The invention provides a method for synthesizing certain of the sulfur containing compounds that is more efficient than previously known methods.
-
Organocatalytic Transformation of Aldehydes to Thioesters with Visible Light作者:Yueteng Zhang、Peng Ji、Wenbo Hu、Yongyi Wei、He Huang、Wei WangDOI:10.1002/chem.201900932日期:2019.6.21A metal‐ and oxidant‐free catalytic method for accessing structurally diverse thioesters from readily accessible, widespread aldehydes, is described. A strategy of a simple organic 9,10‐phenanthrenequinone‐promoted hydrogen atom transfer (HAT) with visible light was successfully implemented to selectively generate acyl radicals without inducing crossover reactivity of thioester products. The preparative
-
Metal‐Free Synthesis of Thiosulfonates via Insertion of Sulfur Dioxide作者:Guoqing Li、Ziyu Gan、Kexin Kong、Xiaomeng Dou、Daoshan YangDOI:10.1002/adsc.201900157日期:2019.4.16A simple and catalyst‐free strategy was developed for the synthesis of unsymmetrical thiosulfonates using readily available DABCO⋅(SO2)2 as a solid and bench‐stable sulfur dioxide surrogate. The corresponding thiosulfonates were obtained through a radical pathway with good functional group tolerance. This strategy offers a promising synthesis method for the construction of diverse and useful thiosulfonates
-
Unsymmetrical disulfide and sulfenamide synthesis via reactions of thiosulfonates with thiols or amines作者:Nobukazu TaniguchiDOI:10.1016/j.tet.2017.02.047日期:2017.4The reactivity of thiosulfonates with nucleophilic reagents was investigated. When reactions of thiosulfonates with thiols were performed, unsymmetrical disulfides were obtained in excellent yields. This procedure could employ numerous aryl or alkyl thiols. On the other hand, reactions of thiosulfonates with amines proceeded in the presence of a copper catalyst. The procedure was performed efficiently
表征谱图
-
氢谱1HNMR
-
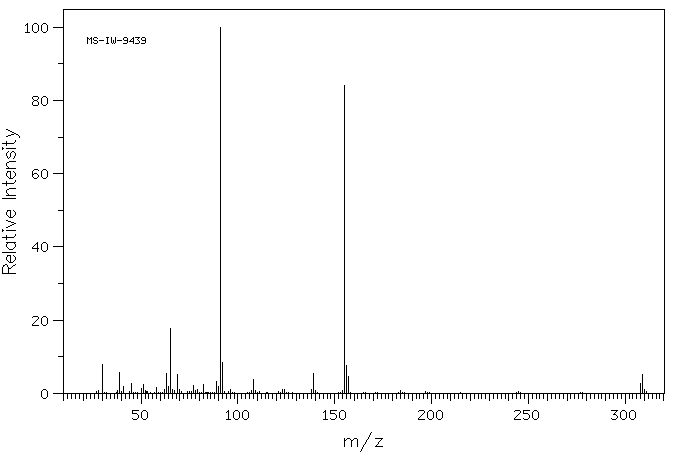
质谱MS
-
碳谱13CNMR
-
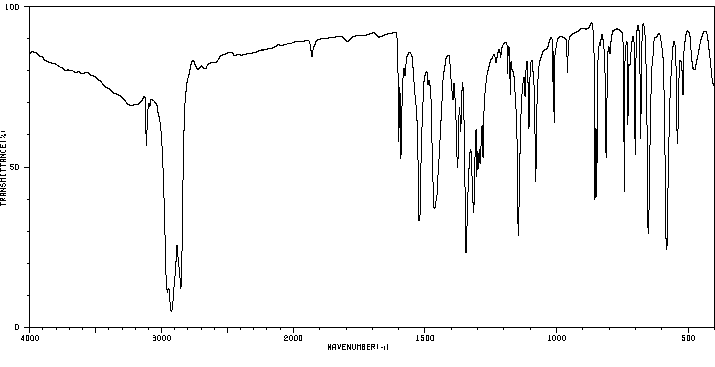
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫