1,3,5,7-tetramethyl-1,3,5,7-tetrasilaadamantane | 17995-33-4
中文名称
——
中文别名
——
英文名称
1,3,5,7-tetramethyl-1,3,5,7-tetrasilaadamantane
英文别名
1,3,5,7-Tetramethyl-tetrasila-adamantan;1,3,5,7-Tetramethyl-1,3,5,7-tetrasila-adamantan;1,3,5,7-tetramethyl-1,3,5,7-tetrasilatricyclo[3.3.1.13,7]decane
CAS
17995-33-4
化学式
C10H24Si4
mdl
——
分子量
256.643
InChiKey
XMNIPURNPMVXKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.58
-
重原子数:14
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— octamethyltrisilmethylene 5695-47-6 C10H28Si3 232.589
反应信息
-
作为反应物:描述:1,3,5,7-tetramethyl-1,3,5,7-tetrasilaadamantane 在 一氯化碘 作用下, 以 二氯甲烷 为溶剂, 反应 45.0h, 生成 trifluoro-methanesulfonic acid 3,5-dimethyl-7-trifluoromethanesulfonyloxy-1,3,5,7-tetrasila-tricyclo[3.3.1.13,7]dec-1-yl ester参考文献:名称:1-Triflato-3,5,7-trimethyl-1,3,5,7-tetrasilaadamantane and 1,3-bis-triflato-5,7-dimethyl-1,3,5,7-tetrasilaadamantane; synthesis, complexation study and X-ray structure of 1-hydroxy-3,5,7-trimethyl-1,3,5,7-tetrasilaadamantane摘要:The symmetrical 1,3,5,7-tetramethyl-1,3,5,7-tetrasilaadamantane (1) reacts with 1.5 molar equivalents of ICl-AgOTf at room temperature to furnish the hitherto unknown 1-triflato-3,5,7-trimethyl-1,3,5,7-tetrasilaadamantane (9) chemoselectively, in near quantitative yields. The treatment of 9 with 2 mol equivalents of ICl-AgOTf affords the bis-triflate 10 in high yields. Control experiments showed that reaction of 1 with ICl (1 mol equivalents) -CCl4 is sluggish, giving 18% conversion to 1-chloro-3,5,7-trimethyl-1,3,5,7-tetrasilaadamantane (2) after 23 h at room temperature. Addition of AlBr3 catalyzes the process, allowing 73%, conversion after 21 h at room temperature. The ICI-AgOTf reagent eliminates the need to prepare and isolate 2 as an intermediate for the synthesis of 9. In silicon NMR spectra, the Si-29 Si-OTf resonance of 10 is slightly upfield compared with that of 9. Treatment of 9 with a saturated solution of B(C6F5)(3) in CH2Cl2 at room temperature shows only minor deshielding at silicon as determined by Si-29-NMR spectroscopy. Attempts to generate donor-acceptor complexes by treatment of 9 with AI(OTf)(3)-CD3CN and with liquid BCl3 gave no notable changes in the Si-OTf NMR chemical shift. In attempts to grow crystals from 9 and 10, white crystals suitable for X-ray analysis were obtained from a sample of 9, the molecular structure of which was shown to be 1-hydroxy-3,5,7-trimethyl-1,3,5,7-tetra-silaadamantane (11), generated by hydrolysis of the Si-OTf bond. (C) 2002 Elsevier Science B.V. All rights reserved.DOI:10.1016/s0022-328x(02)01642-x
-
作为产物:参考文献:名称:Fritz,G. et al., Angewandte Chemie, 1970, vol. 82, p. 445 - 446摘要:DOI:
文献信息
-
Fritz, Gerhard, Angewandte Chemie, 1987, vol. 99, # 11, p. 1150 - 1171作者:Fritz, GerhardDOI:——日期:——
-
Kovacs, Ilona; Magull, Joerg; Fritz, Gerhard, Bulletin de la Societe Chimique de France, 1995, vol. 132, p. 585 - 589作者:Kovacs, Ilona、Magull, Joerg、Fritz, GerhardDOI:——日期:——
-
Reactivity studies of bridgehead organosilicon compounds with nucleophilic reagents作者:G. D. Homer、L. H. SommerDOI:10.1021/ja00804a026日期:1973.11
-
FRITZ, G.;NEUTZNER, J.;VOLK, H., Z. ANORG. UND ALLG. CHEM., 1983, 497, N 2, 21-55作者:FRITZ, G.、NEUTZNER, J.、VOLK, H.DOI:——日期:——
-
FRITZ, G.;HONOLD, J., Z. ANORG. UND ALLG. CHEM., 556,(1988) N 1, 23-56作者:FRITZ, G.、HONOLD, J.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
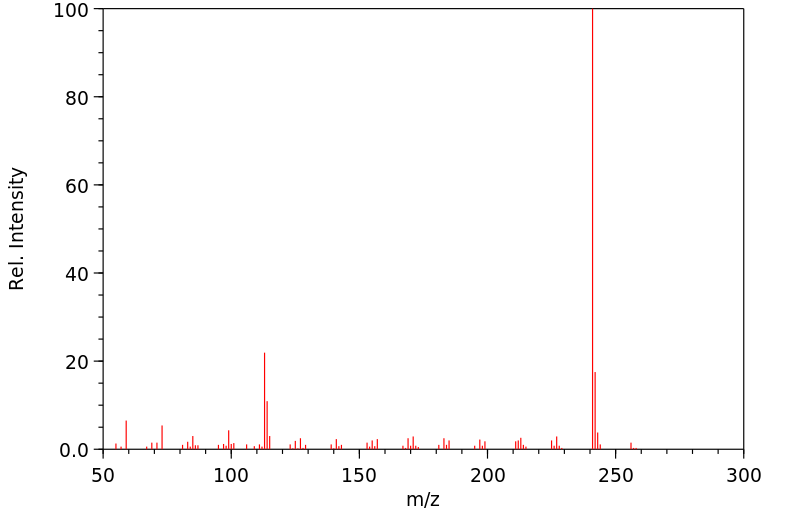
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
()-2-(5-甲基-2-氧代苯并呋喃-3(2)-亚乙基)乙酸乙酯
(双(2,2,2-三氯乙基))
(乙基N-(1H-吲唑-3-基羰基)ethanehydrazonoate)
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
(S)-氨氯地平-d4
(S)-8-氟苯并二氢吡喃-4-胺
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(S)-4-氯-1,2-环氧丁烷
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(S)-2-(环丁基氨基)-N-(3-(3,4-二氢异喹啉-2(1H)-基)-2-羟丙基)异烟酰胺
(SP-4-1)-二氯双(喹啉)-钯
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(R,S)-可替宁N-氧化物-甲基-d3
(R,S)-六氢-3H-1,2,3-苯并噻唑-2,2-二氧化物-3-羧酸叔丁酯
(R)-(+)-5'-苄氧基卡维地洛
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-卡洛芬
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(R)-4-异丙基-2-恶唑烷硫酮
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(N-{4-[(6-溴-2-氧代-1,3-苯并恶唑-3(2H)-基)磺酰基]苯基}乙酰胺)
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6R,7R)-7-苯基乙酰胺基-3-[(Z)-2-(4-甲基噻唑-5-基)乙烯基]-3-头孢唑啉-4-羧酸二苯甲基酯
(6-羟基嘧啶-4-基)乙酸
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(6,6-二甲基-3-(甲硫基)-1,6-二氢-1,2,4-三嗪-5(2H)-硫酮)
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5R,Z)-3-(羟基((1R,2S,6S,8aS)-1,3,6-三甲基-2-((E)-prop-1-en-1-yl)-1,2,4a,5,6,7,8,8a-八氢萘-1-基)亚甲基)-5-(羟甲基)-1-甲基吡咯烷-2,4-二酮
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-(4-乙氧基-3-甲基苄基)-1,3-苯并二恶茂)
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氯-2,1,3-苯并噻二唑-4-基)-氨基甲氨基硫代甲酸甲酯一氢碘
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(5-氨基-1,3,4-噻二唑-2-基)甲醇
(4aS-反式)-八氢-1H-吡咯并[3,4-b]吡啶
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(4S,4''S)-2,2''-环亚丙基双[4-叔丁基-4,5-二氢恶唑]
(4-(4-氯苯基)硫代)-10-甲基-7H-benzimidazo(2,1-A)奔驰(德)isoquinolin-7一
(4-苄基-2-甲基-4-nitrodecahydropyrido〔1,2-a][1,4]二氮杂)
(4-甲基环戊-1-烯-1-基)(吗啉-4-基)甲酮
(4-己基-2-甲基-4-nitrodecahydropyrido〔1,2-a][1,4]二氮杂)
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)