5-溴代乳清酸 | 15018-62-9
中文名称
5-溴代乳清酸
中文别名
5-溴代乳清酸(二水)
英文名称
5-bromo-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
英文别名
5-bromoorotic acid;5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidine-4-carboxylic acid;5-Brom-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-4-carbonsaeure;5-Brom-orotsaeure;5-Bromorotsaeure;5-bromo-2,4-dioxo-1H-pyrimidine-6-carboxylic acid
CAS
15018-62-9
化学式
C5H3BrN2O4
mdl
——
分子量
234.994
InChiKey
YQYHIPBLZSIHDI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:285 °C (decomp)
-
密度:2.187±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下稳定。
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:95.5
-
氢给体数:3
-
氢受体数:4
安全信息
-
WGK Germany:3
-
RTECS号:RM3201000
SDS
制备方法与用途
制备方法
用于生化研究。
用途简介暂无描述。
用途用于生化研究。
反应信息
-
作为反应物:参考文献:名称:Notes. 5-Bromoorotic Acid摘要:DOI:10.1021/jo01103a606
-
作为产物:参考文献:名称:Behrend, Justus Liebigs Annalen der Chemie, 1887, vol. 240, p. 11摘要:DOI:
文献信息
-
A LFER Kinetic Study of The Reaction of 5-Substituted Orotic Acids with Diazodiphenylmethane作者:Fathi H. Assaleh、Aleksandar D. Marinković、Jasmina B. Nikolić、Saša Ž. Drmanić、Danijela Brković、Nevena Prlainović、Bratislav Ž. JovanovićDOI:10.1002/kin.20997日期:2016.7Linear free energy relationships (LFER) were applied to the kinetic data for the reaction of 5‐substituted orotic acids, series 1, with diazodiphenylmethane (DDM) in N,N–dimethylformamide and compared with results obtained for 2‐substituted benzoic acids, series 2. The correlation analysis of the kinetic data with σ substituent parameters was carried out using SSP (single substituent parameter) methods将线性自由能关系(LFER)应用于5取代的乳清酸系列1与N,N-二甲基甲酰胺中的重氮二苯甲烷(DDM)反应的动力学数据,并与2取代的苯甲酸系列所得的结果进行比较2。使用SSP(单取代基参数)方法进行了动力学数据与σ取代基参数的相关性分析。根据比例常数ρ的符号和值,与系列2相比,系列1对取代效应的敏感性较低,为0.876。,1.877。评价取代的“邻效应”是使用Charton模型,其中包括空间取代参数进行,藤田和西冈的模型,该模型描述了总邻-效果的普通极性效应的贡献,邻-位和邻-极性效果。使用Charton模型获得的相关结果显示,对于系列1和2,极性效应的贡献最大,分别为0.861和2.101,而空间效应的重要性最低,分别为0.117和0.055。, 分别。此外,从Fujita-Nishioka模型获得的具有立体效应的系数负值–0.08较低,表明其立体效应较低,影响了系列1中反应速率的降低。
-
Heteroaryl-Fused 2-Phenylisothiazolone Inhibitors of Cartilage Breakdown作者:Stephen W. Wright、Joseph J. Petraitis、Matthew M. Abelman、Douglas G. Batt、Lori L. Bostrom、Ronald L. Corbett、Carl P. Decicco、Susan V. Di Meo、Bruce FreimarkDOI:10.1021/jm00045a012日期:1994.9The synthesis, biological evaluation, and structure-activity relationships of a series of N-phenyl heteroaryl-fused isothiazolones are described. These isothiazolones have been shown to exhibit potent, dose-dependent inhibition of IL-1 beta-induced breakdown of proteoglycan in a cartilage organ culture assay. This effect is likely due to inhibition of MMP activation and a consequent reduction in MMP activity following IL-1 beta stimulation. Thus these compounds potentially represent simple, non-peptidic disease-modifying agents for the treatment of arthritic diseases. To examine the effects of structure on in vitro activity, three general features of the molecules were varied, substituents on the pendant N-phenyl group, the position of ring fusion to the isothiazolone, and substituents on the fused ring peri to the isothiazolone sulfur.
-
AlMe<sub>3</sub>-Promoted Formation of Amides from Acids and Amines作者:Jianqing Li、Krishnananthan Subramaniam、Daniel Smith、Jennifer X. Qiao、Jie Jack Li、Jingfang Qian-Cutrone、John F. Kadow、Gregory D. Vite、Bang-Chi ChenDOI:10.1021/ol203007s日期:2012.1.6In the presence of AlMe3, amines can be directly coupled with acids through dimethylalumlnum amide Intermediates to form the corresponding amides. A wide range of amines and acids including less nucleophilic amines, bulky amines, unprotected secondary amino acids, and acids with poor solubility were coupled smoothly to give the desired products in 55-98% yields.
-
The synthesis of pyrimidineisothiazolones. The effect of temperature on the addition of aryl amines to functionalized pyrimidines.作者:Carl P. Decicco、David J. NelsonDOI:10.1016/s0040-4039(00)61393-x日期:1993.12A new synthesis for the hither-to-unknown pyrimidine isothiazolones 1 and 2 is detailed. The low temperature selectivity of amine nucleophiles on 6-chloropyrimidine-5-acylbromides (12) for the acylbromide over the ring chloride provides a new procedure for the functionalization of 5-carboxypyrimidines.
表征谱图
-
氢谱1HNMR
-
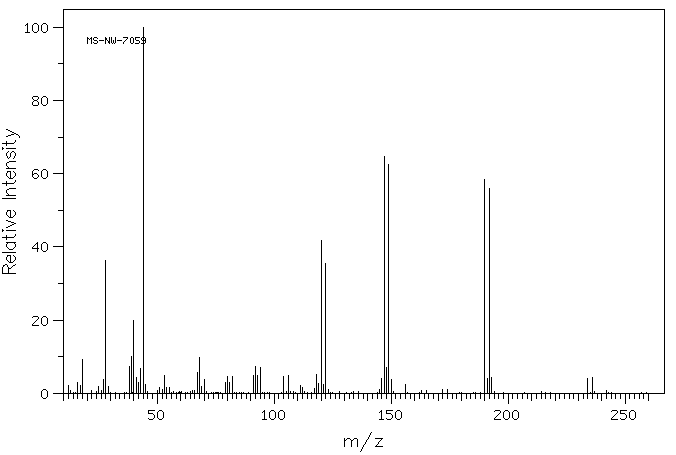
质谱MS
-
碳谱13CNMR
-
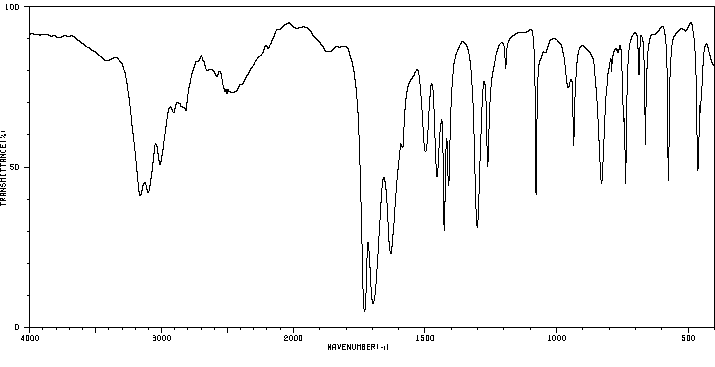
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3