5-辛炔-3-醇 | 53723-18-5
中文名称
5-辛炔-3-醇
中文别名
——
英文名称
(+/-)-5-octyn-3-ol
英文别名
Oct-5-in-3-ol;5-Octyn-3-ol;oct-5-yn-3-ol
CAS
53723-18-5
化学式
C8H14O
mdl
MFCD00041595
分子量
126.199
InChiKey
GGXQZVKQXYOPTF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-39°C (estimate)
-
沸点:74-75°C 12mm
-
密度:0.8537 (estimate)
-
闪点:74-75°C/12mm
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
-
安全说明:S24/25
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Biocatalytic oxidative kinetic resolution of sec-alcohols: stereocontrol through substrate-modification摘要:Whole lyophilised cells of Rhodococcus ruber DSM 44541 were employed for the oxidative kinetic resolution of sec-alcohols using acetone as hydrogen acceptor. The enantioselectivity of this process could be controlled effectively by introducing C-C multiple bonds into substrates, which were inefficiently recognised, in particular short-chain (omega-1)-alcohols and (omega-2)-analogs. Thus, the enantioselectivities of rac-2-pentanol (E= 16.8) and rac-3-octanol (E= 13.3) were significantly improved by introducing a C=C bond adjacent to the alcohol moiety to give racemic (E)-pent-3-en-2-ol and 4-(E)-octen-3-ol, which were resolved with excellent selectivities (E >100 and 50, respectively). In addition, it was found that high stereodifferentiation between the E- and Z-configured double bonds occurred, as the corresponding (Z)-isomers were not converted. Similar selectivity-enhancing effects were observed with acetylenic analogs. (C) 2003 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0957-4166(02)00795-4
文献信息
-
Stereoselective synthesis of 1,2,4-triols via intramolecular bis-silylation of CarbonCarbon triple bonds followed by hydrogenation作者:Masahiro Murakami、Hideaki Oike、Mitsuru Sugawara、Michinori Suginome、Yoshihiko ItoDOI:10.1016/s0040-4020(01)89908-7日期:1993.5new strategy for the stereoselective synthesis of 1,2,4-triols has been developed. An alkyne tethered to a disilanyl group, upon treatment with palladium acetate and tert-alkyl isocyanide, furnished an exocyclic bis-silylated olefin. Subsequent hydrogenation took place from the less-hindered side of the ring producing cis-disubstituted oxasilolane. Oxidation of the two CSi bonds of the hydrogenated
-
Hydrogenation Promoter, Hydrogenation Catalyst, and Process for Producing Alkene Compound申请人:Hori Junichi公开号:US20080033221A1公开(公告)日:2008-02-07A hydrogenation promoter of the present invention is produced by reacting an alkyne compound or an alkene compound, a palladium compound represented by a general formula Pd(II)X j L k (where L represents a monodentate ligand or a polydendate ligand other than a phosphorus-containing ligand (when two or more Ls are present in the compound, the Ls may be the same or different), X represents an anionic group, j represents a value determined according to the valence of X so that X j has a valence of −2 as a whole, and k represents an integer in the range of 0 to 4), and a base in an organic solvent. Specifically, The hydrogenation promoter of the invention includes palladium nanoparticles containing the alkyne compound or the alkene compound as an agglomeration-preventing agent.
-
3-(Alkynylalkyloxy) Cephalosporins申请人:ELI LILLY AND COMPANY公开号:EP0168222A2公开(公告)日:1986-01-153-(Alkynylelkyloxy)cephalosporin antibiotics and the intermediates therefor are disclosed. Pharmaceutical compositions for the antibiotics are also disclosed.本研究公开了 3-(烷基炔基氧基)头孢菌素类抗生素及其中间体。还公开了这些抗生素的药物组合物。
-
Synthesis of Carbocyclic and Heterocyclic Fused Quinolines by Cascade Radical Annulations of Unsaturated <i>N</i>-Aryl Thiocarbamates, Thioamides, and Thioureas作者:Wu Du、Dennis P. CurranDOI:10.1021/ol0344319日期:2003.5.1[GRAPHICS]Tandem radical cyclizations of suitably substituted N-aryl thiocarbamates, thioamides, and thioureas are induced by exposure to tris(trimethylsilyl)silane (TTMSH) and UV light and provide furoquinolines, isofuroquinolines, cyclopentaquinolines, indoloquinollnes, and related ring systems. The intermediacy of an alpha-thioalkylamino radical, which is the synthetic equivalent of an imidoyl radical, is invoked.
-
Enantioselective esterifications of unsaturated alcohols mediated by a lipase prepared from Pseudomonas sp作者:Kevin Burgess、Lee D. JenningsDOI:10.1021/ja00016a032日期:1991.7Competition experiments and measurements of enantioselectivities were used to develop a simple active-site model (Figure 1) for resolutions of beta-hydroxy-alpha-methylene carbonyl compounds III via acyl transfers mediated by lipase from Pseudomonas sp. (AK). Further experiments were used to test and refine this model with respect to resolutions of allylic, propargylic, homopropargylic, and other alcohols (Tables I-IV, respectively). The model proved extremely reliable for predicting the sense of the asymmetric induction, and the combined data collected in this paper give an indication of what structural features of the substrates can be correlated with high enantioselectivities in these resolutions. Furthermore, the results account for the conspicuous reversal of enantioselectivity previously observed in resolutions of gamma-hydroxy-alpha,beta-unsaturated esters 35. Kinetic resolutions of two substrates (allenol 14 and dienol 9) via asymmetric epoxidations were performed for comparison with the methodology presented in this paper.
表征谱图
-
氢谱1HNMR
-
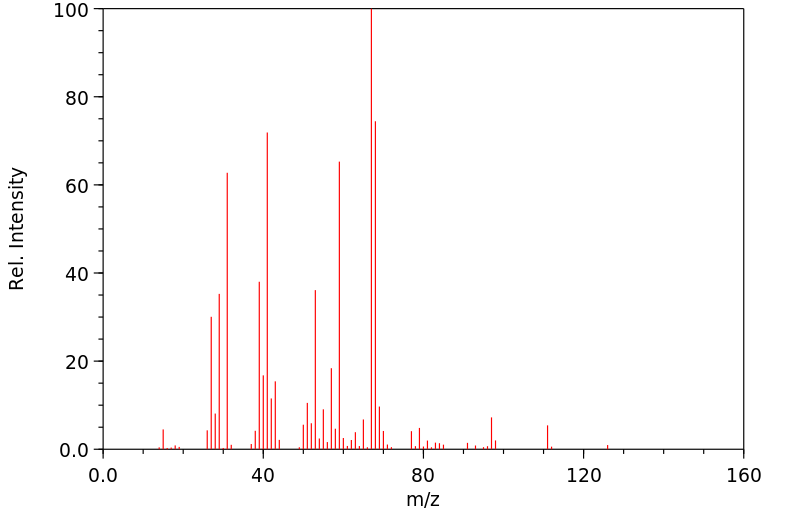
质谱MS
-
碳谱13CNMR
-
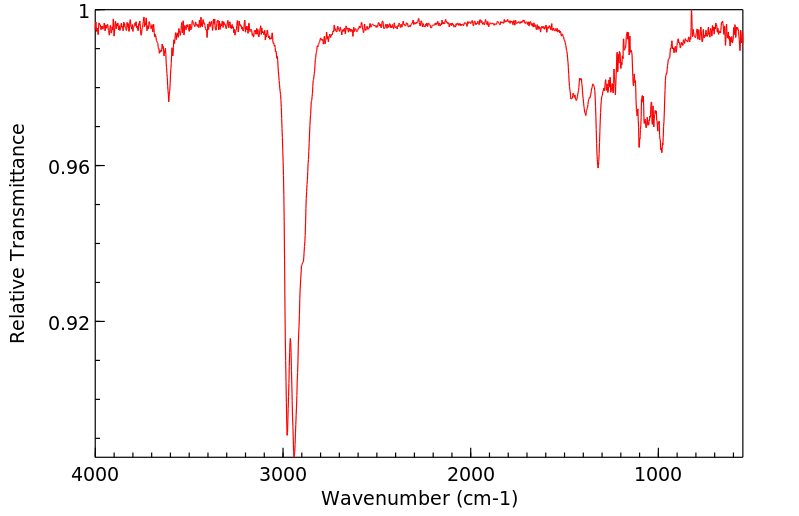
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯