苦味肼 | 653-49-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:386°C (rough estimate)
-
密度:1.7652 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:17
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:176
-
氢给体数:2
-
氢受体数:8
安全信息
-
海关编码:2928000090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,1-二甲基-2-(2,4,6-三硝基苯基)肼 2,2-dimethyl-1-(2,4,6-trinitrophenyl)hydrazine 62054-70-0 C8H9N5O6 271.189 1,2-二(2,4,6-三硝基苯基)肼 2,2',4,4',6,6'-hexanitrohydrazobenzene 68683-32-9 C12H6N8O12 454.226 —— pyruvaldehyde bis-(2,4-dinitro-phenylhydrazone) 1107-69-3 C15H12N8O8 432.309 2-叠氮基-1,3,5-三硝基苯 2-azido-1,3,5-trinitro-benzene 1600-31-3 C6H2N6O6 254.118 (E)-二(2,4,6-三硝基苯基)二氮烯 2.2'.4.4'.6.6'-Hexanitroazobenzol 19159-68-3 C12H4N8O12 452.21
反应信息
-
作为反应物:描述:参考文献:名称:Grandmougin; Leemann, Chemische Berichte, 1906, vol. 39, p. 4384摘要:DOI:
-
作为产物:描述:参考文献:名称:Giua; Cherchi, Gazzetta Chimica Italiana, 1919, vol. 49 II, p. 154摘要:DOI:
文献信息
-
Cytotoxic compounds. Part XIV. Reactions of the bismethanesulphonates of 3-arylthiopropane-1,2-diols and of 2-arylthiopropane-1,3-diols with nucleophiles作者:M. S. Khan、L. N. OwenDOI:10.1039/p19720002060日期:——tetramethylammonium acetate in acetone, though elimination then competes to an increasing extent, becoming the exclusive mode of reaction of the 2,4-dinitro-compounds. With sodium methoxide, elimination is dominant and sometimes is accompanied by allylic rearrangement. All the elimination reactions occur without the intervention of an episulphonium ion. Solvolysis in methanol proceeds under kinetic control and gives在标题反应中形成的产物已通过1 H nmr光谱检查。芳基是对-甲氧基-,对-甲硫基-,p由重排产物的形成所判断的-氯-或2,4-二硝基-苯基和环化为epi砜离子的难易程度依次降低。在所有情况下,乙酸的溶剂分解主要生成1,2-二乙酸盐,这是热力学控制的结果(2,4-二硝基化合物除外,可实现动力学控制)。用乙酸酐中的乙酸钾可形成同分异构的二乙酸酯混合物,其中1,3-化合物的比例随芳基吸电子特性的增加而降低,乙酸四甲基铵在丙酮中也会发生类似的趋势,尽管消除后会竞争越来越多地成为2,4-二硝基化合物的排他性反应方式。对于甲醇钠,消除是主要的,有时伴随着烯丙基重排。所有消除反应均在没有上s离子干预的情况下发生。在甲醇中的溶剂分解在动力学控制下进行,得到1,2-和1,3-二甲基醚的混合物。苯基硫化钠至少在很大程度上通过直接替代来实现S N 2攻击,并且这也发生在两种2,4-二硝基化合物与溴化锂的反应中
-
Investigation of energetic materials prepared by reactions of diamines with picryl chloride作者:Melike Atakol、Arda Atakol、Aynur Özler Yigiter、Ingrid Svoboda、Orhan AtakolDOI:10.1007/s10973-016-5800-4日期:2017.3containing picryl group(s) were synthesized from reactions of hydrazine, 1,2-diaminoethane, 1,3-diaminopropane, 1,4-diaminobutane and 1,7-diaminoheptane with picryl chloride under hydrothermal conditions in methanol. Hydrazine reaction yielded N-2,4,6-trinitrophenylhydrazine which has a single picryl group, whereas the other reactants formed symmetric products with both amine groups connected to picryl groups在甲醇中,在水热条件下,由肼,1,2-二氨基乙烷,1,3-二氨基丙烷,1,4-二氨基丁烷和1,7-二氨基庚烷与氯化吡啶反应,合成了五种含有吡啶基的化合物。肼反应产生具有单个苦味基的N-2,4,6-三硝基苯肼,而其他反应物形成对称的产物,两个胺基均连接到苦味基。这些化合物是N,N'-二-2,4,6-三硝基苯基-1,2-二氨基乙烷,双-N,N'-二-2,4,6-三硝基苯基-1,3-二氨基丙烷,双-N ,N'-二-2,4,6-三硝基苯基-1,4-二氨基丁烷和双-N,N'-二-2,4,6-三硝基苯基-1,7-二氨基庚烷。通过XRD方法揭示了其中两个化合物N-2,4,6-三硝基苯基肼和双-N,N'-di-2,4,6-三硝基苯基-1,3-二氨基丙烷的分子结构。通过TG和DSC方法研究所有化合物。N-2,4,6-三硝基苯肼的热行为具有爆炸性,在极短的温度间隔(180-185°C)中发生强烈爆炸。在其他化
-
An efficient strategy for the preparation of insensitive energetic materials: intramolecular cyclization of picrylhydrazone into an indazole derivative作者:Jie Tang、Jieyi Chen、Pengju Yang、Hongwei Yang、Guangbin ChengDOI:10.1039/d0nj03476b日期:——The intramolecular cyclization reaction of picrylhydrazone into an indazole derivative was systematically studied. The optimized conditions, mechanism and the generality of the cyclization reaction from picrylhydrazone to indazole were investigated. Picrylhydrazones 1a–6a and indazole derivatives 1b and 3b–7b were synthesized through a one-step reaction and the crystal structures of 2a, 4a and 5b were系统地研究了苦瓜into到吲唑衍生物的分子内环化反应。研究了从吡hydr到吲唑环化反应的优化条件,机理和一般性。通过一步反应合成了吡啶甲hydr 1a-6a和吲唑衍生物1b和3b-7b,并通过单晶X射线衍射分析表征了2a,4a和5b的晶体结构。在所有化合物中,吲唑衍生物表现出24–341 ms -1的增加(0.3–4.3%),0.6–3.1 GPa(2.2–14.3%)和9–82°C(5.0–44.0%)的爆震性能和热稳定性以及降低4–10 J(15.4–33.3%) )和50-120 N(16.1-50.0%)的机械敏感性(与甲基吡啶并相比)。此外,通过指纹图谱,Hirshfeld表面和爆炸特性的组合证明了结构与属性的关系。所有这些结果表明,该研究可以丰富高能材料设计的未来前景。
-
Zur Kenntnis der Chrysenchinonphenylhydrazone作者:Ellinor Weiss-Berg、R. WizingerDOI:10.1002/hlca.19570400422日期:——Es wird ermittelt, dass beim 1,2-Chrysenchinon die Arylhydrazonbildung in 2-Stellung erfolgt.Es wird ermittelt,dass beim 1,2-Chrysenchinon和2-Stellung erfolgt中的Arylhydrazonbildung。
-
The structure and some molecular properties of acetone-picrylhydrazone作者:K C Brown、B R Nelson、J W Quail、B E Robertson、J A Weil、Z ZimpelDOI:10.1139/v99-121日期:1999.7.1
Various physical measurements and quantum-mechanical computations to characterize molecular 2-propanone(2,4,6-trinitrophenyl)hydrazone, alias acetone-picrylhydrazine (AH), are reported, including an X-ray diffraction structural determination, an 1H and 13C NMR study of its internal hindered reorientation, and a theoretical (SCF-MO) interpretation of these observations. The structure of AH was determined by X-ray crystallography. The space group is Pbar over 1, with a = 10.1768(9) Å, b = 7.7968(18) Å, c = 8.0018(5) Å, α = 92.102(6)°, β = 99.919(7)°, γ = 105.926(6)°, Z = 2, wR2(F2) = 0.1995 based on all 2748 unique reflections. The (picryl) proton NMR thermal work yielded a Gibbs activation energy ΔG = 46.9 ± 0.4 kJ mol-1 in acetone-d6 and 48.1 ± 0.2 kJ mol-1 in chloroform-d, whereas 13C NMR (two pairs in the picryl ring) yielded 46.6 ± 1.0 and 46.4 ± 1.0 kJ mol-1 in acetone-d6. The SCF-MO computations yielded a detailed model of the conformerization path. Various model conformations and tautomers of AH have been considered, as has removal of H+ or of H0 from its hydrazinic linkage.Key words: dynamic NMR, picrylhydrazone, hindered rotation, activation parameters, SCF-MO model.
报道了各种物理测量和量子力学计算来表征分子2-丙酮肼(2,4,6-三硝基苯肼),别名丙酮-皮酰肼(AH),包括X射线衍射结构测定,其内部受阻转动的1H和13C NMR研究,以及这些观察结果的理论(SCF-MO)解释。通过X射线晶体学确定了AH的结构。空间群为Pbar over 1,a = 10.1768(9)Å,b = 7.7968(18)Å,c = 8.0018(5)Å,α = 92.102(6)°,β = 99.919(7)°,γ = 105.926(6)°,Z = 2,wR2(F2)= 0.1995,基于所有2748个唯一反射。 (皮酰)质子NMR热力学工作在丙酮-d6和氯仿-d中分别产生了Gibbs活化能ΔG‡ = 46.9±0.4 kJ MOl-1和48.1±0.2 kJ MOl-1,而13C NMR(在皮酰环中的两对)在丙酮-d6中产生了46.6±1.0和46.4±1.0 kJ MOl-1。SCF-MO计算得出了构象化路径的详细模型。考虑了AH的各种模型构象和互变异构体,以及从其肼链中去除H+或H0。关键词:动态NMR,皮酰肼,受阻转动,活化参数,SCF-MO模型。
表征谱图
-
氢谱1HNMR
-
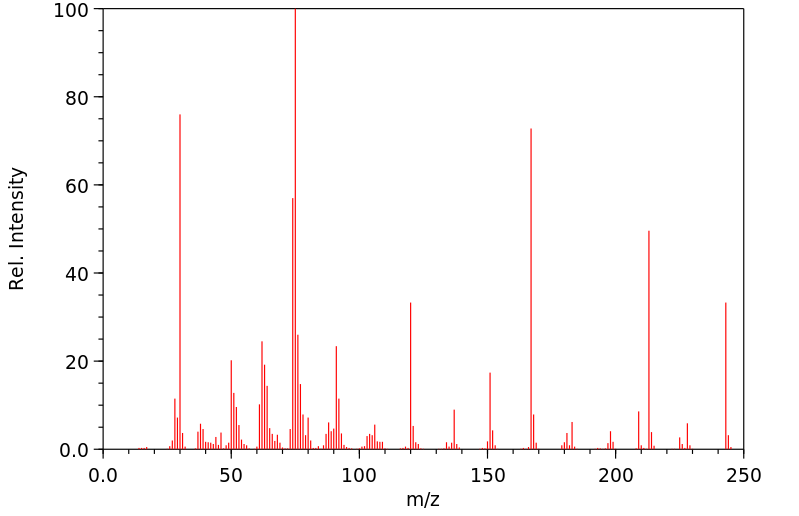
质谱MS
-
碳谱13CNMR
-
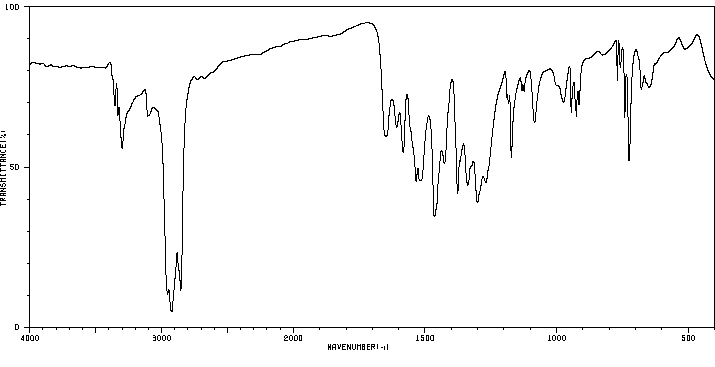
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息