Trp-Gly-Gly | 20762-31-6
中文名称
——
中文别名
——
英文名称
Trp-Gly-Gly
英文别名
WGG;H-Trp-gly-gly-OH;2-[[2-[[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]amino]acetyl]amino]acetic acid
CAS
20762-31-6
化学式
C15H18N4O4
mdl
MFCD00037966
分子量
318.332
InChiKey
JVTHMUDOKPQBOT-NSHDSACASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:787.9±60.0 °C(Predicted)
-
密度:1.397±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-2.1
-
重原子数:23
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.266
-
拓扑面积:142
-
氢给体数:4
-
氢受体数:4
安全信息
-
海关编码:2933990090
反应信息
-
作为反应物:描述:参考文献:名称:与细菌细胞壁粘肽前体相关的肽对125I标记的瑞斯托菌素与黄褐微球菌细胞的结合的抑制作用:定量的构效关系。摘要:已开发了N-Ac氨基酸,N-Ac二肽和N-Ac三肽在抑制125I标记的瑞氏菌素与黄褐微球菌细胞壁结合中的定量构效关系(QSAR),以探索ristocetin之间的结合细节和N-乙酰化的肽 相关方程表明(1)对于C末端氨基酸侧链具有较大摩尔折射率(MR)值的肽,其结合作用更强;(2)对于极性肽,其结合作用较弱。 (3)N-Ac二肽中的N末端氨基酸对结合亲和力的贡献是C末端氨基酸的12倍,DOI:10.1021/jm00121a018
文献信息
-
Linear energy correlations and failures in the low-energy tandem mass spectra of protonatedN-benzoylated tripeptides: Tools for probing mechanisms of CAD processes作者:Daniel G. Morgan、Maurice M. BurseyDOI:10.1002/jms.1190300410日期:1995.4The backbone cleavages for three series of protonated N-benzoyl tripeptide ions were studied in a hybrid tandem mass spectrometer: (i) benzoyl-Gly-Gly-Xxx, where Xxx = Gly, Ala, Val, Leu, Ile, Phe, Tyr, Met, Glu, Pro and Trp, (ii) benzoyl-Gly-Xxx-Gly, where Xxx = Gly, Ala, Leu, Phe, Tyr, Met and Trp, and (iii) benzoyl-Xxx-Gly-Gly, where Xxx = Gly, Ala, Val, Leu, Ile, Phe, Tyr, Met, Pro and Trp. C-Terminal y-type ions and N-terminal a- and b-type ions were noted in all three cases. For benzoyl-Gly-Gly-Xxx, a linear relationship between log (y1/b2) and the proton affinity of the C-terminal amino acid substituents was found: as the proton affinity of the C-terminal residue increases, the fraction of y1 ion formation increases. A similar relationship was noted for the benzoyl-Xxx-Gly-Gly tripeptides between log (y2/b1) and the proton affinity of the N-terminal amino acid substituent: as the proton affinity of the N-terminal residue increases, the fraction of b1 ion formation increases. For the series benzoyl-Gly-Xxx-Gly, these relationships did not hold true. These observations point to similar reaction pathways throughout the benzoyl-Gly-Gly-Xxx series and also similar pathways throughout the benzoyl-Xxx-Gly-Gly, but pathways that are substituent dependent for benzoyl-Gly-Xxx-Gly. The increased correlation coefficients for benzoyl-Gly-Gly-Xxx and benzoyl-Xxx-Gly-Gly when compared with the free tripeptides, suggest that fewer interfering competitive reactions exist, as fewer possibilities for internal hydrogen bonding exist in the N-benzoyl derivatives versus the free compounds.在一台混合串联质谱仪上,研究了三种系列的质子化N-苯甲酰三肽离子的骨架断裂:(i)苯甲酰-Gly-Gly-Xxx,其中Xxx = Gly、Ala、Val、Leu、Ile、Phe、Tyr、Met、Glu、Pro和Trp,(ii)苯甲酰-Gly-Xxx-Gly,其中Xxx = Gly、Ala、Leu、Phe、Tyr、Met和Trp,以及(iii)苯甲酰-Xxx-Gly-Gly,其中Xxx = Gly、Ala、Val、Leu、Ile、Phe、Tyr、Met、Pro和Trp。在所有三种情况下,都观察到了C端y型离子和N端a型及b型离子。对于苯甲酰-Gly-Gly-Xxx,发现log(y1/b2)与C端氨基酸取代基的质子亲和力之间存在线性关系:随着C端残基的质子亲和力增加,y1离子的形成分数增加。对于苯甲酰-Xxx-Gly-Gly三肽,log(y2/b1)与N端氨基酸取代基的质子亲和力之间存在类似的关系:随着N端残基的质子亲和力增加,b1离子的形成分数增加。对于苯甲酰-Gly-Xxx-Gly系列,这些关系并不成立。这些观察结果表明,在苯甲酰-Gly-Gly-Xxx系列和苯甲酰-Xxx-Gly-Gly系列中存在类似的反应途径,但对于苯甲酰-Gly-Xxx-Gly,途径依赖于取代基。与自由三肽相比,苯甲酰-Gly-Gly-Xxx和苯甲酰-Xxx-Gly-Gly的相关系数增加,表明存在的干扰竞争反应较少,因为在N-苯甲酰衍生物中内部氢键的可能性比自由化合物少。
-
NUTRITIVE EMULSION申请人:OTSUKA PHARMACEUTICAL CO., LTD.公开号:EP0312612A1公开(公告)日:1989-04-26The present invention provides a nutritive emulsion characterized in that the emulsion comprises essential components of about 4 to about 15% by weight of an amino acid preparation, about 3 to about 12% by weight of a lipid, about 71.5 to about 93% by weight of water, and about 0.3 to about 3.0% by weight of an emulsifier having an HLB of at least 13 and selected from the group consisting of sucrose fatty acid ester, glycerin fatty acid ester, polyoxyethylene glycerin fatty acid ester and polyoxyethylene sorbitan fatty acid ester, or an emulsifier mixture of said emulsifier and soybean lecithin and in that the emulsion has a pH of about 6.5 to about 8.5.
-
C-TERMINUS AMIDATION ENZYME COMPOSITION, PROCESS FOR ITS PREPARATION AND ITS USE申请人:SHISEIDO COMPANY LIMITED公开号:EP0447547A1公开(公告)日:1991-09-25A C-terminus amidation enzyme composition of serum or blood plasma origin, which acts on a C-terminus glycine adduct represented by formula (I) (wherein A represents a residue other than α-amino or imino group and α-carboxyl group of a natural α-amino acid origin, and X represents a hydrogen atom or a residue of an amino acid derivative bound to the N atom via a carbonyl group) to produce a C-terminus amidation product represented by formula (II) (wherein A and X are as defined above) and glyoxylic acid, is disclosed. A process for preparing the composition and its use are also disclosed.
-
Enzyme participating in c-terminal amidation, and method of preparing same and use thereof申请人:SHISEIDO COMPANY LIMITED公开号:EP0666318A1公开(公告)日:1995-08-09An enzyme catalyzing the conversion of a peptide C-terminal glycine adduct represented by the following formula (I): (wherein A represents a residue other than α-amino group or imino group and α-carboxylic group derived from naturally occurring α-amino acid, X represents hydrogen atom or a residue of an amino acid derivative which is bonded to N atom through carbonyl group) to a peptide C-terminal α-hydroxyglycine adduct represented by the following formula (II): (wherein A and X have the same meanings as above), and further catalyzing the conversion of the peptide C-terminal α-hydroxylglycine adduct represented by the above formula (II) to a C-terminal amidated compound represented by the following formula (III): (wherein A and X have the see meanings as defined above), which comprises culturing host cells transformed with an expression plasmid comprising a DNA coding for said enzyme, and recovering both or either of said enzymes from the culture, characterized in that in said DNA a portion corresponding to the membrane spanning region has been excised.催化下式(I)所代表的肽 C 端甘氨酸加合物转化的酶: (其中 A 代表α-氨基或亚胺基和α-羧基以外的残基,α-氨基或亚胺基和α-羧基来自天然存在的α-氨基酸,X 代表氢原子或通过羰基与 N 原子结合的氨基酸衍生物残基)转化为下式(II)代表的肽 C-末端α-羟基甘氨酸加合物: (其中A和X的含义同上),并进一步催化由上式(II)代表的肽C-末端α-羟基甘氨酸加合物转化为由下式(III)代表的C-末端酰胺化化合物: (其中A和X的含义与上式相同),其中包括培养用包含编码上述酶的DNA的表达质粒转化的宿主细胞,并从培养物中回收上述两种或其中一种酶,其特征在于,在上述DNA中,对应于跨膜区的部分已被切除。
-
Enzyme participating in C-terminal amidation, and method of preparing same and use thereof申请人:SHISEIDO COMPANY LIMITED公开号:EP0884389A1公开(公告)日:1998-12-16An enzyme participating in C-terminal amidation which acts on a peptide C-terminal glycine adduct represented by the following formula (I): (wherein A represents a residue other than α-amino group or imino group and α-carboxylic group derived from naturally occurring α-amino acid, X represents hydrogen atom or a residue of an amino acid derivative which is bonded to N atom through carbonyl group) to form a peptide C-terminal α-hydroxylglycine adduct represented by the following formula (II): (wherein A and X have the same meanings as above, and an enzyme participating in peptide C-terminal amidation of a peptide terminal glycine adduct which acts on a peptide C-terminal α-hydroxylglycine adduct represented by the above formula (II) to form a C-terminal amidated compound represented by the following formula (III): (wherein A and X have the same meanings as described above) a method of producing the same, and the use thereof.
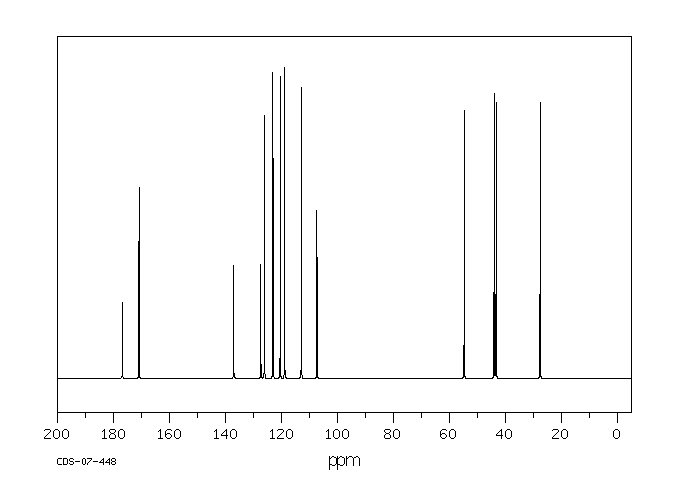
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸