1,2-di(phenylthiomethyl)benzene | 105961-94-2
中文名称
——
中文别名
——
英文名称
1,2-di(phenylthiomethyl)benzene
英文别名
1,2-bis(phenylthiomethyl)benzene;Benzene, 1,2-bis[(phenylthio)methyl]-;1,2-bis(phenylsulfanylmethyl)benzene
CAS
105961-94-2
化学式
C20H18S2
mdl
——
分子量
322.495
InChiKey
LJUAXFBLMKUBNI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:22
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:马来酸酐 、 1,2-di(phenylthiomethyl)benzene 以 乙腈 为溶剂, 以30%的产率得到cis-3a,4,9,9a-tetrahydronaphtho<2,3-c>furan-1,3-dione参考文献:名称:KrF excimer laser photolysis of 1,2-bis(substituted-methyl)benzenes in the presence of alkenes and acetylene; two-photon formation of o-quinodimethane and its cycloaddition with dienophiles摘要:通过 Krf 准分子激光(248 nm)光解 1,2-双(苯氧基甲基)-苯、1,2-双(苯硫甲基)-苯和 1,2-双(苯基硒甲基)-苯的双光子过程,可有效生成邻二甲基喹啉 3;3 与几种二烯烃进行环加成反应,可得到相应的加合物,最大产率为 48%。DOI:10.1039/cc9960002075
-
作为产物:描述:o-Phthalaldehydbis-(diphenylmercaptal) 在 Cp2Ti(P(OEt)3)2 、 二甲基苯基硅烷 作用下, 以41%的产率得到1,2-di(phenylthiomethyl)benzene参考文献:名称:Construction of Extended π-Conjugation Systems Utilizing Novel Multicarbene Complexes of Titanium摘要:钛多卡宾络合物,即具有多种钛-卡宾络合物亚结构的有机钛物质,通过含有空间分离的硫缩醛基团的芳香核与钛烯(II)试剂 Cp2Ti[P(OEt)3]2 反应而易于制备。这些多卡宾络合物与芳香酮反应,产出了多种高度共轭的化合物,并且产率良好。DOI:10.1055/s-2007-984512
文献信息
-
Ionic Liquid as Catalyst and Reaction Medium: A Simple, Convenient and Green Procedure for the Synthesis of Thioethers, Thioesters and Dithianes using an Inexpensive Ionic Liquid, [pmIm]Br作者:Brindaban C. Ranu、Ranjan JanaDOI:10.1002/adsc.200505122日期:2005.11room temperature ionic liquid, 1-pentyl-3-methylimidazolium bromide, [pmIm]Br efficiently catalyzes the reaction of alkyl halides or acyl halides with thiols without any solvent at room temperature leading to the synthesis of thioethers and thioesters in high yields. This reaction has also been extended for the preparation of dithianes and transthioetherification. The ionic liquid is recovered and
-
Half-Sandwich Rhodium/Iridium(III) Complexes Designed with Cp* and 1,2-Bis(phenylchalcogenomethyl)benzene as Catalysts for Transfer Hydrogenation in Glycerol作者:Om Prakash、Kamal Nayan Sharma、Hemant Joshi、Pancham L. Gupta、Ajai K. SinghDOI:10.1021/om500149n日期:2014.5.27glycerol, which acts as a solvent and hydrogen source. Complexes 1–2 are the first examples of Rh species explored for TH in glycerol. The catalysis appears to be homogeneous. The complexes of the (Se, Se) ligand are marginally efficient than the corresponding complexes of the (S, S) ligand. The reactivity of Rh complexes in comparison to those of Ir also appears to be somewhat more. The results of DFT的反应中1,2-双(苯硫基甲基)苯(L1)和1,2-双(phenylselenomethyl)苯(L2)与[(η 5 -Cp *)的MC1(μ-Cl)的] 2(M =铑或IR)在室温下,接着用NH治疗4 PF 6已经导致空气和水分的组合物不敏感半夹心络合物[(η 5 -Cp *)M(大号)CL] [PF 6 ](RH,1 - 2; Ir,3 – 4;L = L1或L2)。他们的HR-MS,1 H,13 C 1H}和77 Se 1 H} NMR光谱被发现是特征性的。的单晶结构1 - 4已经通过X射线晶体学确定。配合物1 - 4已经发现高效的用于在甘油醛和酮的催化转移氢化(TH),其作为溶剂和氢源。复杂1 – 2是在甘油中探索TH的Rh物种的第一个实例。催化作用似乎是均相的。(Se,Se)配体的配合物比(S,S)配体的相应配合物略微有效。与Ir相比,Rh配合物的反应性似乎也更高。DFT
-
Facile Oxidation of Sulfides to Sulfoxides using Sodium Hypochlorite and Oxoammonium Salt as a Catalyst: Chemo- and Diastereoselective Transformation of Bis(phenylthio)alkanes into Sulfoxides作者:Renata Siedlecka、Jacek SkarżewskiDOI:10.1055/s-1994-25486日期:——A facile and selective method for the title transformation is described. The two-phase oxidation of disulfides with one or two equivalents of sodium hypochlorite mediated by TEMPO and co-catalyzed by potassium bromide and PTC leads to the corresponding mono- or disulfoxides, respectively. In the case of 1,2- 1,3-bis(phenylthio)alkanes and ortho-bis(phenylthiomethyl)benzene the respective meso disulfoxides are formed in 90-98% diastereoselectivity.
-
A Facile Synthesis of Tetra- and Dihydronaphthalene Derivatives by Excimer Laser Photolysis of 1,2-Bis(substituted-methyl)benzenes in the Presence of Olefins and Acetylene作者:Akihiko Ouchi、Yoshinori KogaDOI:10.1021/jo970953o日期:1997.10.1laser photolyses of 1,2-bis(phenoxymethyl)benzene (1-O), 1,2-bis[(phenylthio)methyl]benzene (1-S), and 1,2-bis[(phenylseleno)methyl]benzene (1-Se) in acetonitrile solutions via a two-photon process, which was followed by cycloaddition of 3A with several dienophiles-maleic anhydride (4a), dimethyl maleate (4b), dimethyl fumarate (4c), fumaronitrile (4d), and dimethyl acetylenedicarboxylate (4e)-to give corresponding
-
Organic synthesis with α-chlorosulphides. Convenient routes to phenylthioacetals from α-diazoketones and alkyl phenyl sulphides via α-chlorosulphides作者:John P. Cronin、Brid M. Dilworth、M. Anthony McKerveyDOI:10.1016/s0040-4039(00)84093-9日期:1986.1
表征谱图
-
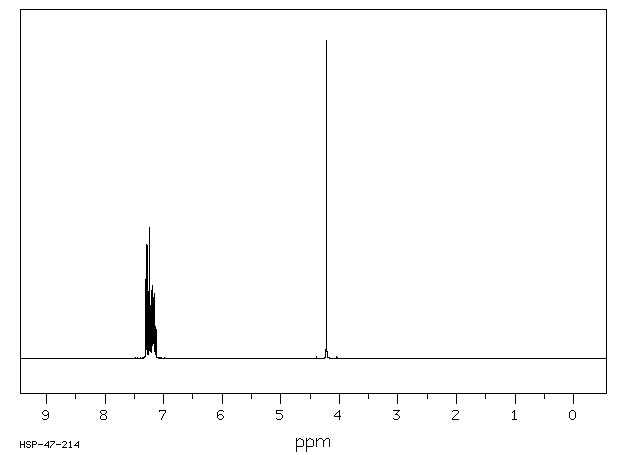
氢谱1HNMR
-
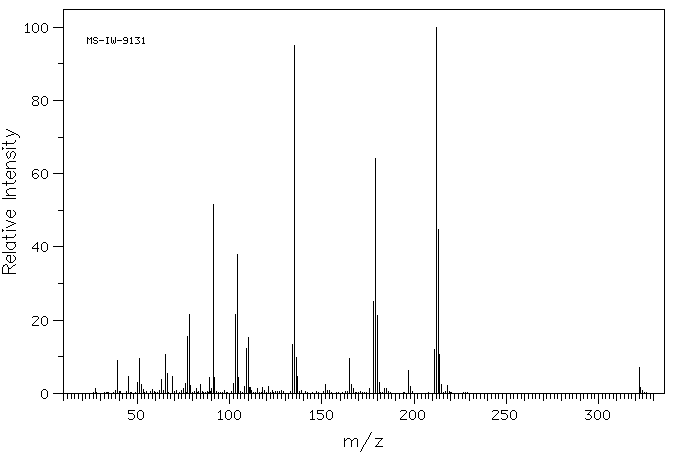
质谱MS
-
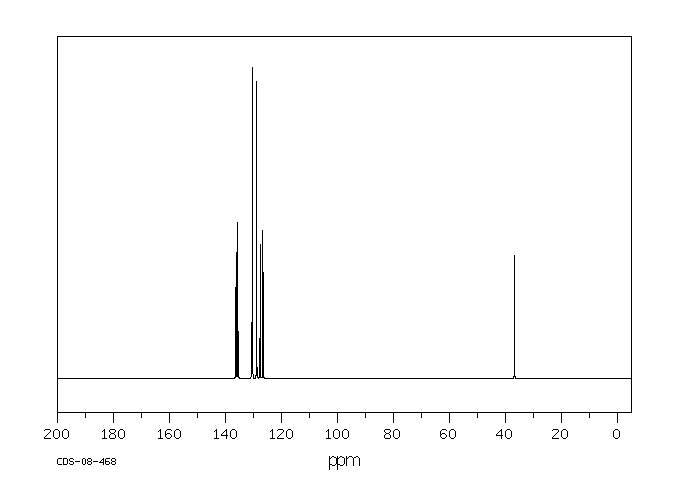
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯