5,5'-difluoro-[1,1'-biphenyl]-2,2'-diol | 388-69-2
中文名称
——
中文别名
——
英文名称
5,5'-difluoro-[1,1'-biphenyl]-2,2'-diol
英文别名
3,3'-Difluor-6,6'-dihydroxybiphenyl;5,5'-difluoro-biphenyl-2,2'-diol;5,5'-Difluor-biphenyl-2,2'-diol;4-Fluoro-2-(5-fluoro-2-hydroxyphenyl)phenol
CAS
388-69-2
化学式
C12H8F2O2
mdl
——
分子量
222.191
InChiKey
PGTVKPNXXXWFMX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:4
反应信息
-
作为产物:描述:4-氟苯酚 在 C27H31ClN5ORu(1+)*F6P(1-) 作用下, 生成 5,5'-difluoro-[1,1'-biphenyl]-2,2'-diol参考文献:名称:Ru(III)-OCl中间体的光谱表征:卤过氧化物酶的结构模拟摘要:卤过氧化物酶利用金属次卤酸盐物质将脂肪族和芳香族 C-H 键卤化为自然界中的 C-X(X = Cl、Br、I)。在这项工作中,我们报告了一种独特的 Ru III -OCl 物种作为卤过氧化物酶的结构模拟物的合成和光谱表征。[(BnTPEN)Ru II (NCCH 3 )] 2+ (BnTPEN = N 1 -苄基-N 1 , N 2 , N 2 -三(吡啶-2-基甲基)乙烷-1,2-二胺)与次氯酸盐在 CH 3 CN : H 2 O 混合物中的酸存在下产生新的 [(BnTPEN)Ru III -OCl]2+种在室温下持续 4.5 小时。这个新物种的特点是紫外-可见吸收、EPR、共振拉曼光谱技术和 ESI-MS。Ru III -OCl 物质能够对各种有机底物进行氧原子转移和氢原子提取。DOI:10.1039/d2dt01947g
文献信息
-
Electrophilic Arene Hydroxylation and Phenol OH Oxidations Performed by an Unsymmetric μ-η1:η1-O2-Peroxo Dicopper(II) Complex作者:Isaac Garcia-Bosch、Xavi Ribas、Miquel CostasDOI:10.1002/chem.201102372日期:2012.2.13affording biphenol coupling products, which is indicative that reactions occur through formation of phenoxyl radicals that then undergo radical CC coupling. Spectroscopic UV/Vis monitoring and kinetic analysis show that reactions take place through reversible formation of ground‐state association complexes [CuII2(μ‐η1:η1‐O2)(X‐PhOH)(m‐XYLN3N4)]2+ (1 O2⋅X‐PhOH) that then evolve through an irreversible的非对称的亚铜反应(II)过氧化氢络合物[铜II 2(μ-η 1:η 1 -O 2)(间- XYL N3N4)] 2+(1 O 2,其中,米-Xyl是heptadentate N基描述了具有酚盐和酚的配体。络合物1 O 2在−90°C下与p ‐X‐PhONa(X = MeO,Cl,H或Me)反应,执行类似酪氨酸酶的邻位酶芳环的羟基化得到相应的邻苯二酚产物。机制研究表明,发生通过初始可逆形成准稳定缔合物的反应物[Cu II 2(μ-η 1:η 1 -O 2)(p -X-PHO)(间- XYL N3N4)] +(1 O 2 ⋅ X-PhO),然后通过过氧化物部分使芳环进行邻羟基化。复杂1分O 2也与发生反应4-X-取代的酚对- X-PhOH(X =的MeO中,Me,F,H或Cl),并用2,4-二-叔丁基苯酚在-90℃下引起的快速衰减1 O 2和得到双酚偶联产物,这表明发生通过形成苯氧自由基,然后进行自由基的C反应
-
One-pot synthesis of dimerized arenes and heteroarenes under mild conditions using Co(<scp>i</scp>) as an active catalyst作者:Adwitiya Pal、Arunabha ThakurDOI:10.1039/d2ob01738e日期:——was achieved by employing 2 mol% of Co(II)catalyst 1 along with Zn dust at room temperature in 2–4 h. The Co(II)/Zn(0) system in situ generates Co(I) as an active catalyst. This catalyst can effectively substitute expensive Pd catalysts and hygroscopic and air-sensitive ZnCl2, generally employed to generate such dimerized heterocyclic cores. Pd is replaced with the Co core and anhydrous ZnCl2 is replaced通过在室温下使用 2 mol% 的 Co( II ) 催化剂1和锌粉,在 2-4 小时内实现了二聚芳烃和杂芳烃系统的可行一锅法合成。Co( II )/Zn(0) 系统原位生成 Co( I ) 作为活性催化剂。这种催化剂可以有效地替代昂贵的 Pd 催化剂和吸湿性和空气敏感的 ZnCl 2,通常用于生成这种二聚杂环核。Pd 被 Co 核和无水 ZnCl 2取代被易于处理且高度经济的活化形式的锌粉所取代。虽然传统方法使用高温和/或更长的反应时间,但我们的合成策略实现了预期的目标,在室温下以适度的反应时间(2-4 小时)取代昂贵的试剂,产品产率高(70-89%)同时。Co( II ) 催化剂1易于合成、经济可行、热稳定且对空气或水分不敏感。该催化剂已通过 EPR、IR、CV 和紫外-可见光谱以及 EDX 研究得到很好的表征。
-
Thia-Fries rearrangement of aryl triflinates to trifluoromethanesulfinylphenols作者:Ximin Chen、Marc Tordeux、Jean-Roger Desmurs、Claude WakselmanDOI:10.1016/s0022-1139(03)00106-4日期:2003.9Aryl trillinates are transformed to trifluoromethanesulfinylphenols in the presence of aluminum trichloride in dichloromethane at room temperature according to a thia-Fries rearrangement process. Rigorous exclusion of oxygen is necessary in order to avoid the formation of 2,2'-dihydroxybiaryls as secondary products. (C) 2003 Elsevier Science B.V. All rights reserved.
-
Highly Chemoselective Ni-Catalyzed Protecting-Group-Free 2,2′-Biphenol Synthesis and Mechanistic Insights作者:Cheng-Yu Long、Hao Chen、Cheng Ma、Bo-Wei Zhao、Shen-Huan Li、Yue Cui、Xinge Yang、Shao-Fei Ni、Xue-Qiang WangDOI:10.1021/acs.orglett.2c01367日期:2022.6.17
-
US6031061A申请人:——公开号:US6031061A公开(公告)日:2000-02-29
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
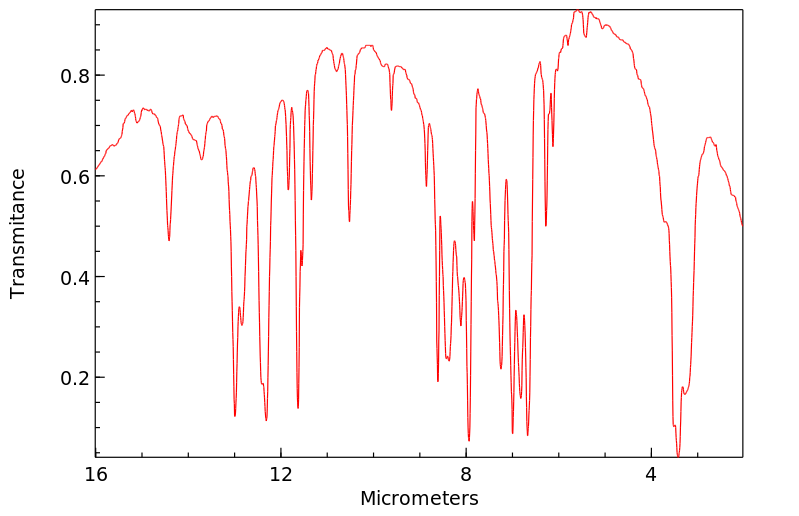
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫