Alpha-吡啶甲酸正丁酯 | 5340-88-5
中文名称
Alpha-吡啶甲酸正丁酯
中文别名
Α-吡啶甲酸正丁酯;α-吡啶甲酸正丁酯
英文名称
butyl pyridine-2-carboxylate
英文别名
butyl picolinate;n-butyl pyridine-2-carboxylate;Picolinsaeure-butylester;n-Butylpicolinat
CAS
5340-88-5
化学式
C10H13NO2
mdl
——
分子量
179.219
InChiKey
HZUPDAXALKWCGX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:13
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:39.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933399090
-
储存条件:请将药品存放在避光、阴凉干燥处,并密封保存。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-吡啶甲酸 2-Picolinic acid 98-98-6 C6H5NO2 123.111
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and structures of 1,2,4-triazoles derivatives摘要:A series of novel 1,2,4-triazole derivatives were synthesized, and their structures were characterized by IR, UV-Vis, FL, NMR, ESI-MS, and elemental analysis. In the meanwhile, the single crystal structures of 3,4-diethyl-5-(4-pyridyl)-1,2,4-triazole and 3,4-dimethyl-5-(o-hydroxyphenyl)-1,2,4-triazole were determined by X-ray diffraction.DOI:10.1134/s1070363215030330
-
作为产物:描述:参考文献:名称:通过布朗斯台德酸活化的N-酰基咪唑鎓盐制备氨基甲酸酯,酯,酰胺和不对称尿素摘要:我们报道了布朗斯台德酸活化的N-酰基咪唑在羰基转化中作为通用中间体的应用。在各种氨基甲酸酯,酯,酰胺和不对称脲上验证了有效且可扩展的程序(21个实例,产率高达91%)。此外,我们以多千克规模为例来说明这种方法,用于合成电子缺陷型氨基甲酸酯。DOI:10.1021/acs.oprd.0c00445
文献信息
-
Practical in situ-generation of phosphinite ligands for palladium-catalyzed carbonylation of (hetero)aryl bromides forming esters作者:Lin Wang、Helfried Neumann、Anke Spannenberg、Matthias BellerDOI:10.1039/c7cc02828h日期:——An effective method for alkoxycarbonylation of (hetero)aryl bromides is developed in the presence of in situ-generated phosphinite ligands tBu2POR (R = nBu, nPr, Et or Me). For this purpose commercially available tBu2PCl was used as the pre-ligand in the presence of different alcohols. For the first time cross coupling reactions with two alcohols – one generating the ligand, the other used as substrate
-
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group作者:Yuji Nakatani、Yuichiro Koizumi、Ryu Yamasaki、Shinichi SaitoDOI:10.1021/ol800576w日期:2008.5.1The conversion of ketones to esters has been achieved through the use of Cu catalyst and tetrabutylammonium nitrite. This reaction involves the activation of the less activated C-C bond, and the alkyl group is removed as a leaving group. Various isopropyl ketones are found to be good substrates for this reaction.
-
Efficient Palladium-Catalyzed Alkoxycarbonylation of <b><i>N</i></b>-Heteroaryl Chlorides - A Practical Synthesis of Building Blocks for Pharmaceuticals and Herbicides作者:Matthias Beller、Wolfgang Mägerlein、Adriano Indolese、Christine FischerDOI:10.1055/s-2001-14576日期:——The alkoxycarbonylation of various N-heteroaryl chlorides was examined in detail. Studies of the butoxycarbonylation of 2- and 3-chloropyridine revealed the importance of selecting both the right phosphine ligand and ligand concentration in order to obtain efficient conversion and selectivity. Amongst the different ligands tested, 1,4-bis(diphenylphosphino)butane (dppb) and 1,1′-bis(diphenylphosphino)ferrocene (dppf) led to the most efficient palladium catalyst systems for the conversion of 2- and 4-chloropyridines and similar heteroaryl chlorides. The best catalytic systems for the alkoxycarbonylation of less activated substrates, such as 3-chloropyridines, were found to be those containing 1,4-bis(dicyclohexylphosphino)butane. Good to excellent yields of a number of N-heterocyclic carboxylic acid esters were realized by applying the appropriate ligand in the right concentration at low catalyst loadings (0.005-0.5 mol% Pd). For the first time catalyst turnover numbers (TON) of up to 13,000 were obtained for the carbonylation of a (hetero)aryl chloride.详细研究了各种N-杂芳基氯化物的烷氧羰基化反应。对2-和3-氯吡啶的丁氧羰基化研究发现,为了获得高效的转化率和选择性,选择合适的膦配体及其浓度至关重要。在测试的不同配体中,1,4-双(二苯基膦)丁烷(dppb)和1,1'-双(二苯基膦)二茂铁(dppf)为2-和4-氯吡啶及类似杂芳基氯化物提供了最高效的钯催化体系。对于活性较低的底物,如3-氯吡啶,最佳催化体系是含有1,4-双(二环己基膦)丁烷的体系。通过在低催化负载量(0.005-0.5摩尔% 钯)下使用适当浓度的合适配体,实现了多种N-杂环羧酸酯的良好至优异产率。对于(杂)芳基氯化物的羰基化反应,首次获得了高达13,000的催化剂周转数(TON)。
-
[EN] ISOTHIAZOLE-PYRIDINE DERIVATIVES AS MODULATORS OF HIF (HYPOXIA INDUCIBLE FACTOR) ACTIVITY<br/>[FR] DÉRIVÉS D'ISOTHIAZOLE-PYRIDINE EN TANT QUE MODULATEURS DE L'ACTIVITÉ DU HIF (FACTEUR INDUCTIBLE PAR L'HYPOXIE)申请人:FIBROGEN INC公开号:WO2009089547A1公开(公告)日:2009-07-16The present invention relates to novel compounds according to Formula I or II, methods, and compositions capable of decreasing HIF hydroxylase enzyme activity, thereby increasing the stability and/or activity of hypoxia inducible factor (HIF). Formula (I) or (II).本发明涉及根据公式I或II的新化合物、方法和组合物,能够降低HIF羟基酶的酶活性,从而增加缺氧诱导因子(HIF)的稳定性和/或活性。公式(I)或(II)。
-
Efficient Carbonylation of Aryl and Heteroaryl Bromides using a Palladium/Diadamantylbutylphosphine Catalyst作者:Helfried Neumann、Anne Brennführer、Peter Groß、Thomas Riermeier、Juan Almena、Matthias BellerDOI:10.1002/adsc.200606044日期:2006.7A general palladium-catalyzed alkoxycarbonylation of aryl and heteroaryl bromides has been developed in the presence of bulky monodentate phosphines. Studies of the butoxycarbonylation of three model substrates revealed the advantages of di-1-adamantyl-n-butylphosphine compared to other ligands. In the presence of this catalyst system various bromoarenes provided the corresponding benzoic acid derivatives
表征谱图
-
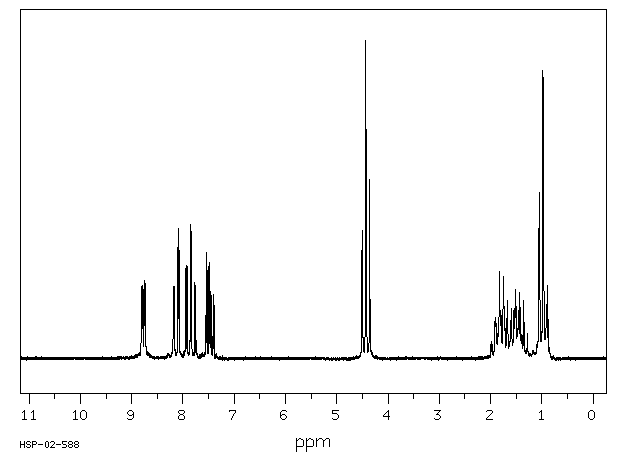
氢谱1HNMR
-
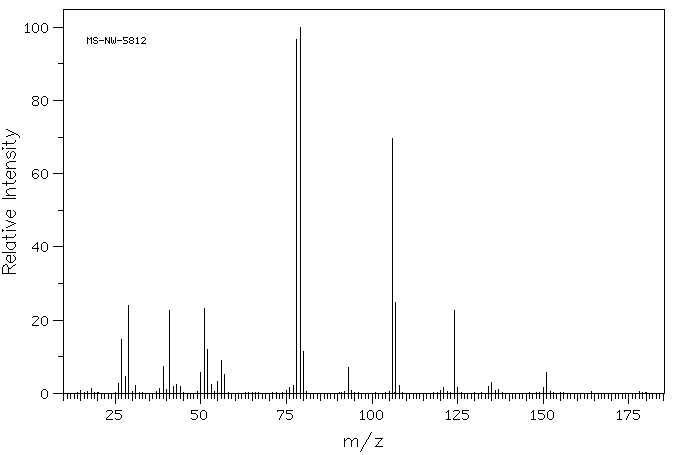
质谱MS
-
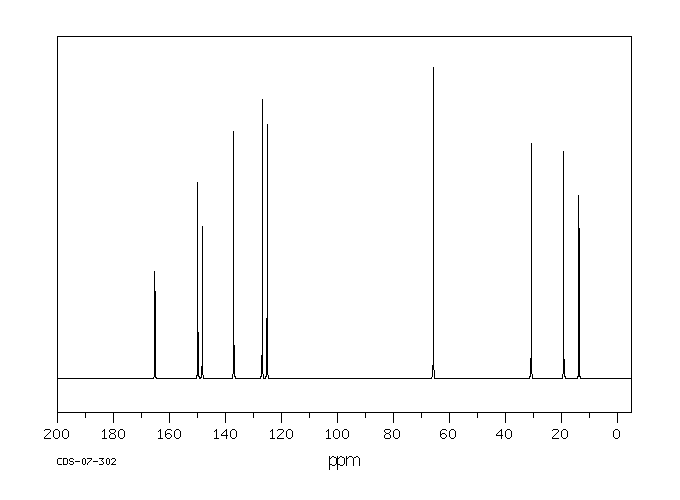
碳谱13CNMR
-
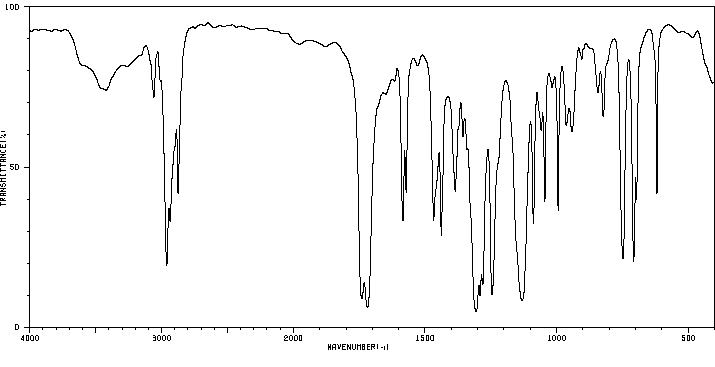
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-