N,N-二甲基邻苯二胺 | 2836-03-5
中文名称
N,N-二甲基邻苯二胺
中文别名
2-氨基-N,N-二甲基苯胺; N,N-二甲基邻苯二胺
英文名称
N,N-dimethyl-1,2-phenylenediamine
英文别名
N1,N1-dimethylbenzene-1,2-diamine;2-(N,N-dimethylamino)aniline;N,N-dimethyl-o-phenylenediamine;N,N-Dimethyl-o-phenylendiamin;2-Dimethylaminoanilin;N,N-dimethyl-1,2-benzenediamine;N,N-dimethylbenzene-1,2-diamine;2-Amino-N,N-dimethyl-anilin;2-amino-N,N-dimethylaniline;2-(dimethylamino)aniline;N1,N1-Dimethylbenzene-1,2-diamine;2-N,2-N-dimethylbenzene-1,2-diamine
CAS
2836-03-5
化学式
C8H12N2
mdl
MFCD01706706
分子量
136.197
InChiKey
HJXIRCMNJLIHQR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:240.49°C (rough estimate)
-
密度:1.049
-
保留指数:1216
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:29.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
海关编码:2921590090
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P501,P273,P272,P260,P270,P202,P201,P271,P264,P280,P391,P308+P311,P361+P364,P333+P313,P301+P312+P330,P302+P352+P312,P304+P340+P311,P305+P351+P338+P310,P403+P233,P405
-
危险品运输编号:3082
-
危险性描述:H311+H331,H302,H318,H361,H373,H317
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Amino-N,N-dimethylaniline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Amino-N,N-dimethylaniline
CAS number: 2836-03-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H12N2
Molecular weight: 136.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Amino-N,N-dimethylaniline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Amino-N,N-dimethylaniline
CAS number: 2836-03-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H12N2
Molecular weight: 136.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-isothiocyanato-N,N-dimethylaniline —— C9H10N2S 178.258 —— 3-fluoro-N,N-dimethyl-o-phenylenediamine —— C8H11FN2 154.187 N1,N1-二甲基-N2-苯基苯-1,2-二胺 N1,N1-dimethyl-N2-phenylbenzene-1,2-diamine 26037-46-7 C14H16N2 212.294 —— 2,2'-bis(dimethylamino)azobenzene 14954-74-6 C16H20N4 268.362 N1-(2-(二甲基氨基)苯基)-N2,N2-二甲基-1,2-苯二胺 H(Me)NN2 1072901-09-7 C16H21N3 255.363
反应信息
-
作为反应物:描述:N,N-二甲基邻苯二胺 在 potassium dichromate 、 aluminum(III) sulphate octadecahydrate 、 sodium thiosulfate 、 zinc(II) chloride 作用下, 以 水 为溶剂, 反应 2.5h, 以46%的产率得到2-amino-5-dimethylaminophenylthiosulphonic acid参考文献:名称:一种钙敏感受体蛋白拮抗剂衍生的分子探针及在甲状旁腺切除手术中的应用摘要:本发明公开了一种钙敏感受体蛋白拮抗剂衍生的分子探针及在甲状旁腺切除手术中的应用。具体为手性钙敏蛋白抑制剂拮抗剂Calhex 231衍生的含有如通式A1所示的荧光基团的分子探针或含有如通式A2所示的生色团的分子探针;所述通式A1和通式A2的结构如下所示:或者公开号:CN113173891A
-
作为产物:描述:N,N-二甲基对硝基苯胺 在 palladium 10% on activated carbon 、 氢气 作用下, 以 甲醇 为溶剂, 20.0 ℃ 、405.33 kPa 条件下, 反应 4.0h, 生成 N,N-二甲基邻苯二胺参考文献:名称:Synthesis of benzimidazoles via iridium-catalyzed acceptorless dehydrogenative coupling摘要:苯并咪唑类化合物通过铱催化的受体无氧脱氢偶联反应,产率较高。DOI:10.1039/c5ob00904a
-
作为试剂:参考文献:名称:4-phenoxy-nicotinamide or 4-phenoxy-pyrimidine-5-carboxamide compounds摘要:本发明涉及以下式子的新型苯酰胺或吡啶酰胺衍生物: 其中A1、A2、B1、B2和R1到R11如描述和权利要求中所定义,并且其药学上可接受的盐。这些化合物是GPBAR1激动剂,可用作治疗二型糖尿病等疾病的药物。公开号:US08420647B2
文献信息
-
[EN] 4-PHENOXY-NICOTINAMIDE OR 4-PHENOXY-PYRIMIDINE-5-CARBOXAMIDE COMPOUNDS<br/>[FR] COMPOSÉS DE 4-PHÉNOXYNICOTINAMIDE OU DE 4-PHÉNOXY-PYRIMIDINE-5-CARBOXAMIDE申请人:HOFFMANN LA ROCHE公开号:WO2011089099A1公开(公告)日:2011-07-28This invention relates to novel phenyl amide or pyridyl amide derivatives of the formula (I), wherein A1, A2, B1, B2 and R1 to R11 are as defined in the description and in the claims, as well as pharmaceutically acceptable salts thereof. These compounds are GPBAR1 agonists and can be used as medicaments for the treatment of diseases such as type II diabetes.
-
Transition-Metal-Free Access to Primary Anilines from Boronic Acids and a Common <sup>+</sup>NH<sub>2</sub> Equivalent.作者:Samantha Voth、Joshua W. Hollett、J. Adam McCubbinDOI:10.1021/jo5025078日期:2015.3.6are obtained at refluxing temperatures. Our method is also amenable to electrophilic amination of several common boronic acid derivatives (e.g., pinacol esters). We demonstrate that it can be combined with metal–halogen exchange reactions or a variety of directed ortho metalation protocols in a “one-pot” sequence for the synthesis of aromatic amines with unique substitution patterns. DFT studies, in通过使用常见的廉价亲电氮源(H 2 N-OSO 3H,HSA)。通过这种方法发现富含电子的底物具有最高的反应性。但是,在室温条件下,即使是中等电子贫乏的基材也能很好地耐受。与不受阻碍的基材相比,受阻的基材似乎同样有效。高度缺乏电子的底物在室温下以非常低的产率提供产物,但是在回流温度下获得中等至良好的产率。我们的方法也适用于几种常见的硼酸衍生物(例如频哪醇酯)的亲电胺化反应。我们证明了它可以与金属-卤素交换反应或各种定向的邻位金属化方案以“一锅”顺序结合使用,以合成具有独特取代模式的芳香胺。DFT研究,结合实验结果,表明该反应是通过碱介导的HSA活化发生的,然后发生1,2芳基B–N迁移。这种活化方式对于反应的成功似乎至关重要,并且首次允许在环境温度下对硼酸进行一般的亲电子胺化。
-
Visible-Light-Induced Nickel-Catalyzed Cross-Coupling with Alkylzirconocenes from Unactivated Alkenes作者:Yadong Gao、Chao Yang、Songlin Bai、Xiaolei Liu、Qingcui Wu、Jing Wang、Chao Jiang、Xiangbing QiDOI:10.1016/j.chempr.2019.12.010日期:2020.3Transition-metal-catalyzed cross-coupling reactions between naturally abundant sp3-hybridized carbon centers facilitate access to diverse molecules with complex three-dimensional structures. Organometallic compounds are among one of the most powerful reagents that are broadly used in carbon–carbon bond formations. Although sp2-hybridized organometallic compounds are widely employed in cross-couplings, sp3-hybridized自然丰富的sp 3-杂化碳中心之间的过渡金属催化交叉偶联反应有助于获得具有复杂三维结构的各种分子。有机金属化合物是最强大的试剂之一,广泛用于碳-碳键的形成。尽管sp 2-杂化的有机金属化合物广泛用于交叉偶联,但是sp 3-杂化的有机金属偶联剂的开发较少。在这里,我们报告可见光诱导的单个镍催化的C(sp 3)–C(sp 3),C(sp 3)–C(sp 2)和C(sp 3)–C(sp)使用烷基锆茂的交叉偶联反应,该反应很容易从末端或内部未活化的烯烃通过加氢锆和链步反应就地生成。该方法温和,适用于多种底物,包括伯,仲,叔烷基,芳基,烯基,炔基卤化物和各种烯烃。机理研究表明,镍催化的自由基交叉偶联是一种新颖的途径,代表了锆锆茂的首次可见光诱导的转变。
-
[EN] BRUTON'S TYROSINE KINASE INHIBITORS<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON申请人:BIOGEN IDEC INC公开号:WO2011029046A1公开(公告)日:2011-03-10The present invention provides compounds useful as inhibitors of Btk, compositions thereof, and methods of using the same.本发明提供了用作Btk抑制剂的化合物、其组合物以及使用方法。
-
A new facile, efficient synthesis and structure peculiarity of quinoxaline derivatives with two benzimidazole fragments作者:Vakhid A. Mamedov、Nataliya A. Zhukova、Victor V. Syakaev、Aidar T. Gubaidullin、Tat'yana N. Beschastnova、Dil'bar I. Adgamova、Aida I. Samigullina、Shamil K. LatypovDOI:10.1016/j.tet.2012.10.045日期:2013.1A highly efficient and versatile method for the synthesis of quinoxaline derivatives with two benzimidazole fragments have been developed on the basis of the ring contraction of 3-(benzimidazo-2-yl)quinoxalin-2(1H)-one with 1,2-diaminobenzene and its various types of substituted and condensed derivatives. Owing to the inter- and intramolecular processes, involving self association, proton exchange
表征谱图
-
氢谱1HNMR
-
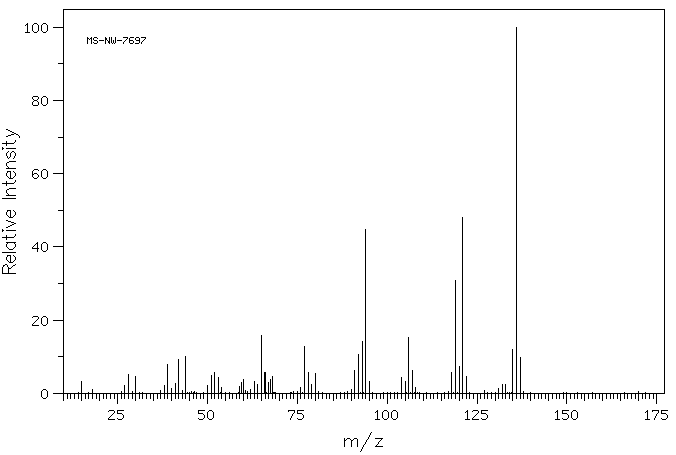
质谱MS
-
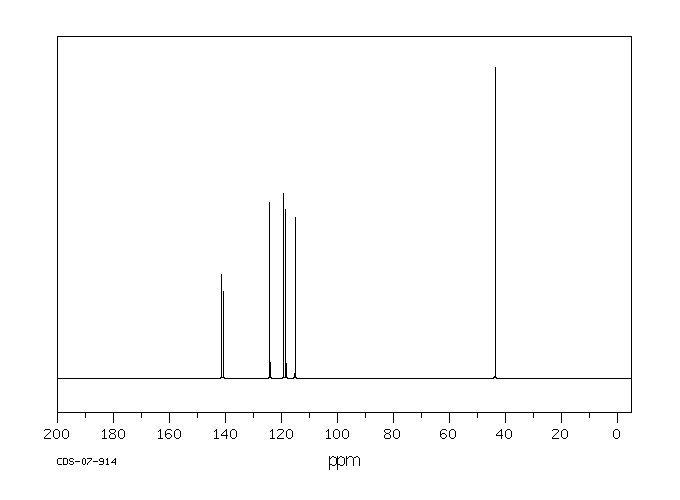
碳谱13CNMR
-
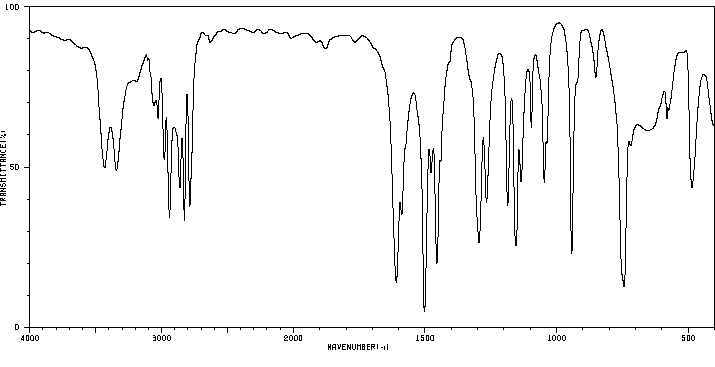
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷