N-(2,6-二甲基苯基)-4-硝基-苯甲酰胺 | 64594-44-1
中文名称
N-(2,6-二甲基苯基)-4-硝基-苯甲酰胺
中文别名
——
英文名称
4-Nitro-N-(2,6-dimethylphenyl)benzamide
英文别名
N-(2,6-dimethylphenyl)-4-nitrobenzamide;4-nitro-benzoic acid-(2,6-dimethyl-anilide);4-Nitro-benzoesaeure-(2,6-dimethyl-anilid);N-(2,6-dimethylphenyl)-4-nitro-benzamide
CAS
64594-44-1
化学式
C15H14N2O3
mdl
MFCD00034008
分子量
270.288
InChiKey
WBLNADQPUSRQIV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:193-196 °C
-
沸点:357.8±42.0 °C(Predicted)
-
密度:1.270±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:20
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:74.9
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2924299090
SDS
上下游信息
反应信息
-
作为反应物:描述:N-(2,6-二甲基苯基)-4-硝基-苯甲酰胺 在 palladium on activated charcoal 硫酸 、 环己烯 、 sodium nitrite 作用下, 以 异丙醇 为溶剂, 反应 8.5h, 生成 4-Hydroxy-N-(2,6-dimethylphenyl)benzamide参考文献:名称:Anticonvulsant Activity and Interactions with Neuronal Voltage-Dependent Sodium Channel of Analogues of Ameltolide摘要:Fifteen compounds related to ameltolide (LY 201116) were studied for (i) anticonvulsant potential in the maximal electroshock-induced seizures (MES) and the subcutaneous pentylenetetrazol (sc Ptz) tests in mice and rats and (ii) interactions with neuronal voltage-dependent sodium channels. Compounds were chosen ranging in anticonvulsant activity in mice from very active to inactive. The active compounds were defined as those protecting 50% of the animals at doses between 10 and 50 mu mol/kg and inactive compounds as those protecting 50% of the animals at doses greater than 1 mmol/kg. The series studied included three N-(2,6-dimethylphenyl)benzamides (compounds 1, 2 (ameltolide), and 3), three N-(2,2,6,6-tetramethyl)piperidinyl-4-benzamides (compounds 4, 5, 6), one phenylthiourea (compound 7), five N-(2,6-dimethylphenyl)phthalimides (compounds 8, 9, 10, 13, and 14), two N-phenylphthalimide derivatives (compounds 11 and 12), and one N-(2,2,6,6-tetramethyl)piperidinyl-4-phtalimide (compound 15). Phenytoin (PHT) was employed as the reference prototype antiepileptic drug. After inital screening in mice, compounds 1, 2, 3, 5, 8, 9, 10, 13, and 14 were selected for further testing in rats. Anticonvulsant ED(50)s (effective doses in at least 50% of animals tested) of compounds in the MES test were determined in rats dosed orally and amounted to 52 (1), 135 (2), 284 (3), 31 (8), 131 (9), 25 (10), 369 (13), 354 (14), and 121 (PHT) mu mol/kg, compound 5 presenting with an ED50 value higher than 650 mu mol/kg. In our hands, the apparent IC(50)s (inhibitory concentrations 50) of compounds toward binding to rat brain synaptosomes of [H-3]batrachotoxinin-A-20 alpha-benzoate were 0.25 (1), 0.97 (2), 0.35 (3), 25.8 (5), 161.3 (8), 183.5 (9), 0.11 (10), 1.86 (13), 47.8 (14), and 0.86 (PHT) mu M. The relationship between the activity in the MES test and the capacity to interact in vitro with neuronal voltage-dependent sodium channels and the fact that the IC50 values obtained in the in vitro test are close to the brain concentrations at which anticonvulsant activities are reported to occur for ameltolide strongly suggest that the anticonvulsant properties of most compounds tested could be a direct result of their interaction with the neuronal voltage-dependent sodium channel.DOI:10.1021/jm9608772
-
作为产物:描述:参考文献:名称:五元杂芳环稠合嘧啶衍生物:设计,合成和刺猬信号通路抑制研究。摘要:一系列新颖的五元杂芳环稠合嘧啶衍生物,包括嘌呤,吡咯并[2,3- d ]嘧啶,吡咯并[3,2- d ]嘧啶,噻吩并[2,3- d ]嘧啶,噻吩并[3, 2- d ]嘧啶和呋喃[3,2- d已经确定]嘧啶是刺猬蛋白信号传导途径的有效抑制剂。描述了这些化合物的合成和SAR。在这一新的刺猬信号通路抑制剂系列中,与vismodegib相比,大多数化合物均表现出显着的抑制活性,这表明五元杂芳环稠合嘧啶在当前报道的刺猬信号通路抑制剂的结构骨架中脱颖而出,成为令人鼓舞的支架,值得更多关注探索和进一步调查。DOI:10.1016/j.bmcl.2014.05.066
文献信息
-
Synthesis and anticonvulsant activity of some 4-nitro-N-phenylbenzamides作者:V Bailleux、L Vallée、JP Nuyts、G Hamoir、JH Poupaert、JP Stables、J VamecqDOI:10.1016/0223-5234(96)88254-7日期:1995.1A short series of 4-nitro-N-phenylbenzamides was synthesized and evaluated for anticonvulsant properties and neurotoxicity. In mice dosed intraperitoneally, three of the four 4-nitro-N-phenylbenzamides were efficient in the maximal electroshock-induced seizure (MES) test, especially N-(2,6-dimethylphenyl)-4-nitrobenzamide (ED(50) value in the MES test = 31.8 mumol/kg, TD(50) = 166.9 mumol/kg, protective合成了简短的4-硝基-N-苯基苯甲酰胺系列,并评估了其抗惊厥性质和神经毒性。在腹膜内给药的小鼠中,四种4-硝基-N-苯基苯甲酰胺中的三种在最大电击诱发癫痫发作(MES)测试中有效,尤其是N-(2,6-二甲基苯基)-4-硝基苯甲酰胺(ED(50)值在MES测试中= 31.8摩尔/千克,TD(50)= 166.9摩尔/千克,保护指数[PI] = 5.2)和N-(2-氯-6-甲基苯基)-4-硝基苯甲酰胺(ED(50)值在MES测试中= 90.3摩尔/千克,TD(50)= 1.068摩尔/千克,PI = 11.8)。还发现后者的4-硝基-N-苯基苯甲酰胺对皮下戊四烯(sc Ptz)引起的癫痫发作具有活性,并被选择用于口服给药的大鼠中作进一步评估。在这些条件下,在MES测试中发现N-(2-氯-6-甲基苯基)-4-硝基苯甲酰胺
-
Intramolecular charge transfer with N-benzoylaminonaphthalenes. 1-Aminonaphthalene versus 2-aminonaphthalene as electron donors作者:Xuan Zhang、Chun-Hua Liu、Li-Hong Liu、Fang-Ying Wu、Lin Guo、Xiang-Ying Sun、Chao-Jie Wang、Yun-Bao JiangDOI:10.1039/b210106h日期:2003.2.11derivatives in cyclohexane and was assigned to the CT state by the observation of a substantial red shift with increasing solvent polarity or with increasing electron-withdrawing ability of the substituent. The CT emission energies were found to follow a linear relationship with the Hammett constant of the substituent and the value of the linear slope for 1-NBAs (-0.45 eV) was higher than that of 2-NBAs (-0制备了在苯甲酰基苯环的对位或间位具有不同取代基的N-(取代-苯甲酰基)-1-氨基萘和N-(取代-苯甲酰基)-2-氨基萘(1-NBA和2-NBA)使用类苯甲酰苯胺电荷转移作为探针反应,探测1-氨基萘(1-AN)和2-氨基萘(2-AN)之间的差异。对于在环己烷中所有制备的氨基萘衍生物,发现了异常的长波发射,并且通过观察到随着溶剂极性的增加或取代基的吸电子能力的增加而发生的大幅度红移,将其分配为CT状态。发现CT发射能量与取代基的Hammett常数和1-NBA(-0。45 eV高于2-NBA(-0.35 eV),后者与苯胺衍生物(BAs,-0.345 eV)接近。这表明在1-NBA的CT状态下,电荷分离的程度更高,其中完全放电分离是通过CT发射能量的线性下降率为-1.00的降低电势依赖性建立的。通过观察发现,当对位,间位和对位时,苯甲酰基取代的BA的相应线性斜率保持不变,从而排除了1-NBA和2
-
Anticonvulsant method and formulations
-
Bourhim, Mustapha; Poupaert, Jacques H.; Stables, James P., Arzneimittel-Forschung/Drug Research, 1999, vol. 49, # 2, p. 81 - 87作者:Bourhim, Mustapha、Poupaert, Jacques H.、Stables, James P.、Vallee, Louis、Vamecq, JosephDOI:——日期:——
-
Design, synthesis and structure–activity relationship of new HSL inhibitors guided by pharmacophore models作者:Jumana D. Al-Shawabkeh、Afaf H. Al-Nadaf、Lina A. Dahabiyeh、Mutasem O. TahaDOI:10.1007/s00044-013-0616-2日期:2014.1Hormone-sensitive lipase (HSL) is a critical enzyme involved in the hormonally regulated release of fatty acids and glycerol from adipocyte lipid stores. Its inhibition may improve insulin sensitivity and blood glucose handling in type 2 diabetes. Accordingly, many small-molecule HSL inhibitors have recently been identified. In continuation of our efforts for discovery of new HSL inhibitors, we prepared a variety of esters, amides, sulfonamides and sulfonate esters capable of fitting two pharmacophore models that we developed and published earlier. The tested compounds were synthesized via coupling reactions of aroyl chlorides or sulfonyl chlorides with phenols, amines and related derivatives. Our efforts led to the identification of interesting compounds of low micromolar anti-HSL bioactivities, which have potential to be developed into effective antidiabetic agents.
表征谱图
-
氢谱1HNMR
-
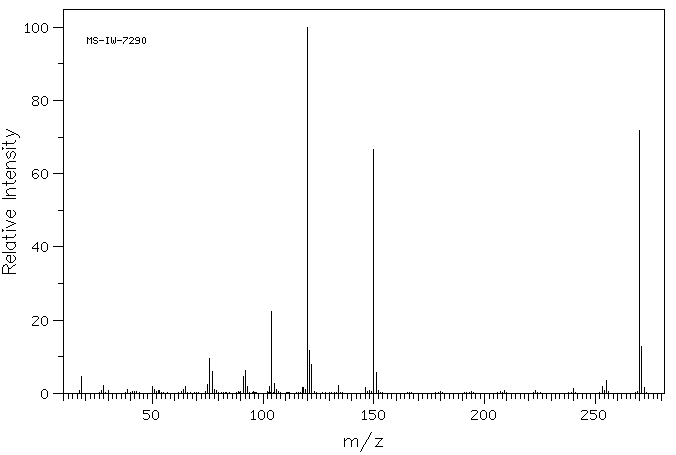
质谱MS
-
碳谱13CNMR
-
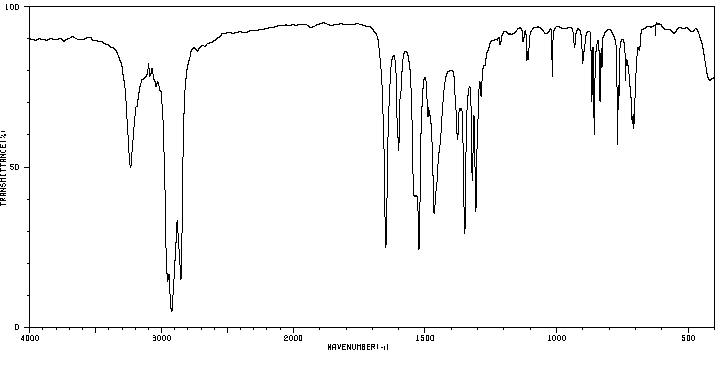
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫