茜素紫 | 2103-64-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>300°C
-
沸点:389.44°C (rough estimate)
-
密度:1.2392 (rough estimate)
-
溶解度:溶解性几乎不溶于水、苯、氯仿;溶于乙醇、丙酮
-
最大波长(λmax):526nm
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:27
-
可旋转键数:0
-
环数:5.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:116
-
氢给体数:4
-
氢受体数:7
安全信息
-
海关编码:2932999099
-
储存条件:2-8°C
SDS
| Name: | Gallein 1-Hydrate Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 2103-64-2 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2103-64-2 | Spiroisobenzofuran-1(3h),9'-9hxanthen- | 100 | 218-272-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2103-64-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C20H12O7.H2O
Molecular Weight: 382.32
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2103-64-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Spiroisobenzofuran-1(3h),9'-9hxanthen-3-one,3',4',5',6'-tetr - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2103-64-2: No information available.
Canada
CAS# 2103-64-2 is listed on Canada's DSL List.
CAS# 2103-64-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2103-64-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
Gallein 是一种 G 蛋白 βγ (Gβγ) 亚基信号抑制剂。它能够干扰 Gβγ 亚基与 PI3Kγ 的相互作用,并具有抗肿瘤活性。此外,Gallein 还可用作红色染料、酸碱指示剂和磷酸盐的检测试剂。
化学性质含1.5分子结晶水的Gallein 棕红色结晶或粉末;不含结晶水则为绿黄色。它在约180℃时失去结晶水,在此温度以上会变黑,但直到300℃也不会熔化。该物质溶于醇、丙酮及稀碱溶液,微溶于乙醚,几乎不溶于水、苯和氯仿。在强酸介质中呈现黄色,在微酸性中呈玫瑰红色,在中性介质中则呈淡紫色,在强碱性介质中先变为紫色再转变为黄色。
用途Gallein 可用作酸碱指示剂(pH3.8(棕黄)-6.6(玫瑰红)),络合指示剂,用于滴定铋和锆、测定尿中磷酸盐。此外,它与四价锡反应呈鲜红色,是检测锡的灵敏试剂。
生产方法Gallein 可由连苯三酚与苯酐通过以下步骤制备:将148g 苯酐和126g 连苯三酚缓慢加热至液化后,在搅拌下继续升温至190-200℃,反应4-5小时。直至反应物浓稠无法搅拌时停止加热。冷却后用热水洗涤产物,并加入10% 氢氧化铵溶液溶解滤去不溶物。将滤液酸化以析出棕色沉淀,然后温水洗涤并使用氨水分结晶一次,最终得到约100g 成品。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(3,4,5,6-tetrahydroxy-xanthen-9-yl)-benzoic acid 54750-05-9 C20H14O7 366.327
反应信息
-
作为反应物:描述:参考文献:名称:Baeyer, Chemische Berichte, 1871, vol. 4, p. 556摘要:DOI:
-
作为产物:参考文献:名称:荧光素和酚酞衍生物内在生物活性的合成与评价摘要:已经合成了荧光素和酚酞衍生物,并通过分子对接、细胞毒性和抗氧化研究筛选了它们的生物活性。在分子对接研究中,化合物 3d 在与 t-RNA 二甲基烯丙基转移酶对接时表现出更好的滑动分数和氢键结合能力。通过 DPPH 和 ABTS 自由基清除活性评估抗氧化能力。在本次筛选中,化合物 3d 在 DPPH 和 ABTS 方法中表现出更好的抑制效率。通过针对人宫颈癌细胞系 (HeLa) 的细胞可持续性测定评估化合物的细胞毒性。所有合成的化合物都表现出对 HeLa 细胞的细胞毒作用,化合物 3d 表现出比标准药物(阿霉素)更好的活性(IC50)。DOI:10.1007/s13738-021-02389-4
文献信息
-
Solvent-Free Synthesis of Sulfonephthaleins, Sulfonefluoresceins and Fluoresceins Under Microwave Irradiation
-
Ferric hydrogensulphate as a recyclable catalyst for the synthesis of fluorescein derivatives作者:Hossein Eshghi、Narges MirzaieDOI:10.2478/s11696-011-0024-3日期:2011.1.1
Abstract Polycondensation reactions of phenols with phthalic anhydride were carried out in the presence of ferric hydrogensulphate under melt conditions. The reactions proceeded in short reaction times by using a catalytic amount of Fe(HSO4)3 and the corresponding fluorescein derivatives were obtained in high yields. The simplicity, scale-up, along with the use of an inexpensive, non-toxic, recyclable catalyst of an environmentally benign nature, are other remarkable features of the procedure. The absorption and emission properties of these fluorescein derivatives were studied.
-
High purity phthalein derivatives and method for preparing same申请人:Tran-Guyon Joanne公开号:US20060106234A1公开(公告)日:2006-05-18The invention concerns high purity phthalcin derivatives enabling their use for medical applications or in the field of biotechnology, as well as their preparation method whereby a phthatic anhydride derivative is condensed with a naphthol or phenol derivative in an organic acid ester and the crystals of the resulting condensate are converted by action of a strong acid or one of its precursors in anhydrous medium.
-
[EN] A NOVEL POLYMER, A COMPOSITION, A METHOD AND A KIT FOR WHITENING TEETH<br/>[FR] NOUVEAU POLYMÈRE, COMPOSITION, PROCÉDÉ ET KIT POUR BLANCHIR LES DENTS申请人:UNILEVER NV公开号:WO2020001864A1公开(公告)日:2020-01-02The present invention relates to a novel polymer for use in an oral care composition for whitening teeth. The invention also relates to a process to prepare the polymer and a kit that contains the composition and a source of light. The polymer is characterized by including a xanthene dye moiety attached therein.本发明涉及一种新型聚合物,用于口腔护理组合物中美白牙齿。该发明还涉及制备聚合物的过程以及包含组合物和光源的套件。该聚合物的特点是包含一个联结在其中的黄色染料基团。
-
Hydrogen peroxide and peracetic acid indicators and methods申请人:3M Innovative Properties Company公开号:US20030194346A1公开(公告)日:2003-10-16The present invention provides a hydrogen peroxide indicator and a peracetic acid indicator that include a substrate on which is disposed an indicator composition that includes at least one of a select group of colorants and a transition metal salt. As a result of exposure to hydrogen peroxide and/or peracetic acid, the colorants change color, and even become colorless, thereby providing an indication of the presence of hydrogen peroxide and/or peracetic acid.
表征谱图
-
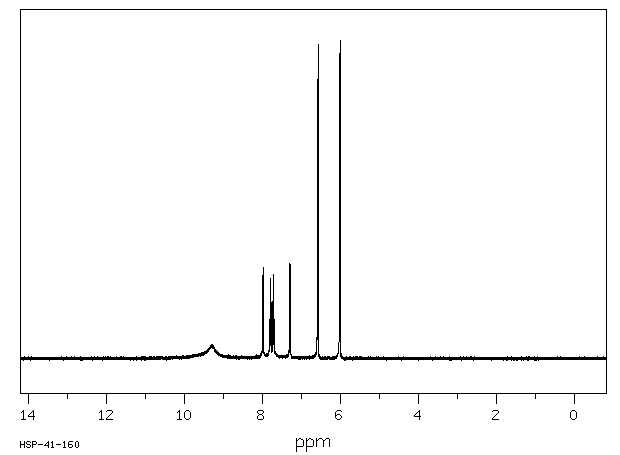
氢谱1HNMR
-
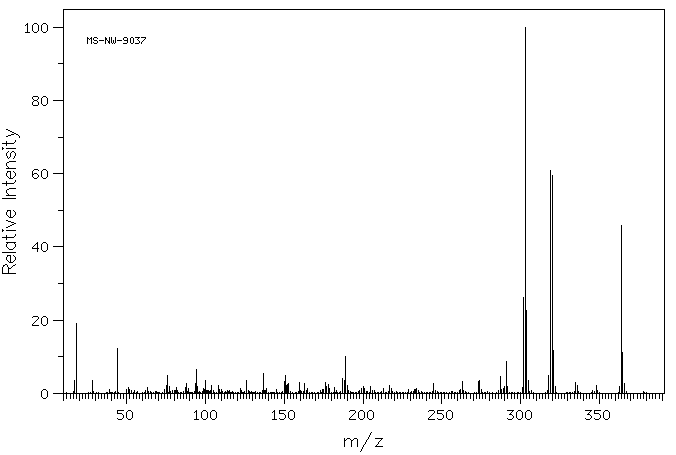
质谱MS
-
碳谱13CNMR
-
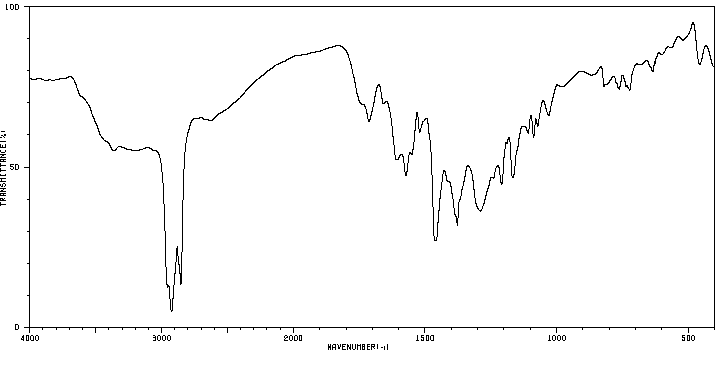
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息