N-亚磺酰-4-氯苯胺 | 13165-68-9
中文名称
N-亚磺酰-4-氯苯胺
中文别名
1-氯-4-(苯亚磺酰基氨基)苯
英文名称
N-(4-chloro-phenyl)-sulfur imide oxide
英文别名
4-Chlor-N-sulfinyl-anilin;N-Sulfinyl-p-chloranilin;p-Chlor-N-sulfinylanilin;4-Chlor-1-sulfinylamino-benzol;p-Chlor-thionylanilin;((4-chlorophenyl)imino)-λ4-sulfanone;p-chloro-N-sulphinylaniline;4-chloro-N-sulfinylaniline;4-Chlor-thionylanilin;Benzenamine, 4-chloro-N-sulfinyl-;1-chloro-4-(sulfinylamino)benzene
CAS
13165-68-9
化学式
C6H4ClNOS
mdl
MFCD00054490
分子量
173.623
InChiKey
GTKDDSPQJMLGOM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:36 °C(Solv: carbon tetrachloride (56-23-5))
-
沸点:102-106 °C(Press: 12 Torr)
-
密度:1.37±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:30.4
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
反应信息
-
作为反应物:描述:N-亚磺酰-4-氯苯胺 在 sodium hydride 作用下, 以 四氢呋喃 、 乙二醇二甲醚 为溶剂, 反应 5.25h, 生成 N-(4-chlorophenyl)-N-methylethenesulfinamide参考文献:名称:Baudin, Jean-Bernard; Commenil, Marie-Gabrielle; Julia, Sylvestre A., Bulletin de la Societe Chimique de France, 1996, vol. 133, # 4, p. 329 - 350摘要:DOI:
-
作为产物:描述:参考文献:名称:钯催化的 1,2,3-氧噻唑烷-2-氧化物的区域选择性 [3+2] 成环策略摘要:据报道, N-亚磺酰基苯胺与乙烯基碳酸亚乙酯和乙烯基环氧化物在钯催化下进行[3+2]环化反应,在温和的反应条件下得到1,2,3-恶噻唑烷-2-氧化物。 1,2,3-恶噻唑烷-2-氧化物在制备亚砜、β-氨基醇和胺中的进一步合成应用也已得到证实。DOI:10.1002/ejoc.202301153
文献信息
-
Di-2-pyridyl sulfite. A new useful reagent for the preparation of N-sulfinylamines, nitriles, isocyanides, and carbodiimides under mild conditions作者:Sunggak Kim、Kyu Yang YiDOI:10.1016/s0040-4039(00)84413-5日期:1986.1Di-2-pyridyl sulfite is a very useful reagent for the preparation of N-sulfinylamines, nitriles, isocyanides, and carbodiimides in high yields under essentially neutral conditions.
-
N-Heterocyclic Carbene Catalysis: Enantioselective Formal [2+2] Cycloaddition of Ketenes and N-Sulfinylanilines作者:Teng-Yue Jian、Lin He、Cen Tang、Song YeDOI:10.1002/anie.201102488日期:2011.9.19Sultam of swing: Both enantiomers of 1,2‐thiazetidin‐3‐one oxides were obtained in very good yields with excellent enantioselectivities when using N‐heterocyclic carbene catalysts (see scheme; M.S.=molecular sieves, TBS=tert‐butyldimethylsilyl). The products were easily converted into 3‐oxo‐β‐sultams, α‐mercapto amides, and β‐mercapto amines through oxidation or reduction.
-
Synthesis, characterization and vibrational studies of p-chlorosulfinylaniline作者:Doly M. Chemes、Diego J. Alonso de Armiño、Edgardo H. Cutin、Heinz Oberhammer、Norma L. RoblesDOI:10.1016/j.molstruc.2016.06.067日期:2017.1obtained liquid compound were studied by Raman and infrared spectroscopy in the liquid state. The assignment of the vibrational spectra was carried out with the help of data obtained by quantum chemical calculations at the harmonic oscillator approximation and using anharmonic vibrational self-consistent field (VSCF) method as well. The 1H and 13C NMR chemical shifts of the molecule were calculated by
-
Cycloaddition von N-sulfinylaminen an ketene作者:H. Beecken、F. KorteDOI:10.1016/s0040-4020(01)99310-x日期:1962.1Aromatic and aliphatic N-sulphinyl amines add smoothly to diphenyl and biphenylene ketene forming substituted 1,2-thiazetidinone-(3)-oxides-(1). An addition of ketene itself to N-sulphinyl cyclohexyl amine can be concluded from secondary products only.
-
New facile synthesis of N-sulfinylamine derivatives using N,N′-sulfinylbisimidazole and N-(chlorosulfinyl)imidazole作者:Hae Kim Yong、Moo Shin JaiDOI:10.1016/s0040-4039(00)89260-6日期:1985.1Treatment of amine derivatives such as amines, sulfonamides, and amides with N,N′-sulfinylbisimidazole and N-(chlorosulfinyl)imidazole respectively gives the corresponding N-sulfinylamine derivatives (); the latter reaction using N-(chlorosulfinyl)imidazole yields in almost quantitative yields at 20°C under mild conditions.
表征谱图
-
氢谱1HNMR
-
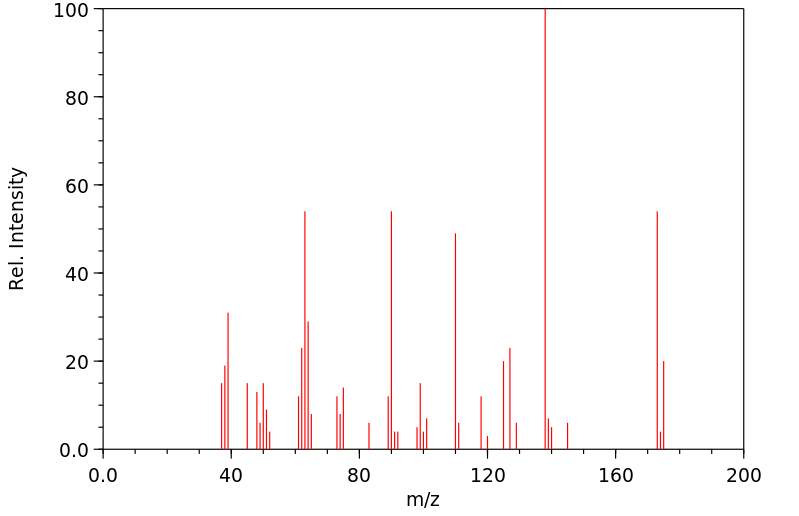
质谱MS
-
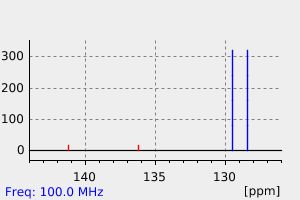
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫