2,4,7-trimethyl-1,8-naphthyridine | 14757-44-9
中文名称
——
中文别名
——
英文名称
2,4,7-trimethyl-1,8-naphthyridine
英文别名
——
CAS
14757-44-9
化学式
C11H12N2
mdl
——
分子量
172.23
InChiKey
WJJNPDSYYWZSBC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:13
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:2,4,7-trimethyl-1,8-naphthyridine 在 正丁基锂 、 重水 作用下, 以 四氢呋喃 、 正己烷 为溶剂, 生成 2,7-Bis(deuteriomethyl)-4-methyl-1,8-naphthyridine参考文献:名称:Regioselective Addition ofn-Alkyllithiums to α,α′-Disubstituted-1,8-Naphthyridines: Synthesis of 6-Amino-3-Pyridinol Analogs of α-Tocopherol摘要:在非极性溶剂(如 Et2O-己烷混合物)中,将正烷基锂加入到δ,δ′-二取代的-1,8-萘啶中。在极性溶剂(如四氢呋喃)中,烷基锂充当碱而不是亲核体。对于能够与(烷基)锂试剂进行五元环螯合的底物,可实现区域选择性加成。以 TBS 保护醇作为共螯合基团的底物具有最佳的产率和区域选择性。这种方法被成功用于制备五甲基色醇(PMC)的两种 6-氨基-3-吡啶醇类似物,PMC 是一种δ-生育酚衍生物,其异戊烯侧链被截断为一个甲基。DOI:10.1055/s-2005-865308
文献信息
-
BIOMOLECULE BINDING LIGANDS申请人:Lowe Christopher Robin公开号:US20100203650A1公开(公告)日:2010-08-12The invention provides biomolecule binding ligands, collections of biomolecule binding ligands, and their use in the purification of biological mixtures and in the identification of ligands having an affinity for a substance. The ligand is a compound of formula (III) or a compound of formula (IV): wherein for compounds of formula (I) one of R 1a , R 1b , R 2 , R 3 and R 4 is a group comprising a linker attached to a support, and the others of R 1a , R 1b , R 2 , R 3 and R 4 are independently selected from optionally substituted C 1-20 alkyl, optionally substituted C 3-20 heterocyclyl or optionally substituted C 5-20 aryl, and R 1a , R 1b and R 2 are additionally selected from hydrogen, and R 2 is additionally further selected from —S(═O)R 5 and —C(═S)NR 6 R 7 , wherein R 5 , R 6 and R 7 are independently optionally substituted C 1-20 alkyl, optionally substituted C 3-20 heterocyclyl or optionally substituted C 5-20 aryl, or, optionally, two or more of the others of R 1a , R 1b , R 2 , R 3 and R 4 , together with the atoms to which they are bound, may form a ring; and for compounds of formula (II) one of R 1a , R 1b , R 3 and R 4 is a group comprising a linker attached to a support, and the others of R 1a , R 1b , R 3 and R 4 are independently selected optionally substituted C 1-20 alkyl, optionally substituted C 3-20 heterocyclyl or optionally substituted C 5-20 aryl, and R 1a , and R 1b are additionally selected from hydrogen, or, optionally, two or more of the others of R 1a , R 1b , R 3 and R 4 , together with the atoms to which they are bound, may form a ring.
-
[EN] BIOMOLECULE BINDING LIGANDS<br/>[FR] LIGANDS SE LIANT À DES BIOMOLÉCULES申请人:CAMBRIDGE ENTPR LTD公开号:WO2009007676A2公开(公告)日:2009-01-15The invention provides biomolecule binding ligands, collections of biomolecule binding ligands, and their use in the purification of biological mixtures and in the identification of ligands having an affinity for a substance. The ligand is a compound of formula (III) or a compound of formula (IV): wherein for compounds of formula (I) one of R1a, R1b,R2, R3 and R4 is a group comprising a linker attached to a support, and the others of R1a, R1b,R2, R3 and R4 are independently selected from optionally substituted C1-20 alkyl, optionally substituted C3-20 heterocyclyl or optionally substituted C5-20 aryl, and R1a, R1b and R2 are additionally selected from hydrogen, and R2 is additionally further selected from -S(=O)R5 and -C(=S)NR6R7, wherein R5, R6 and R7 are independently optionally substituted C1-20 alkyl, optionally substituted C3-20 heterocyclyl or optionally substituted C5-20 aryl, or, optionally, two or more of the others of R1a, R1b,R2, R3 and R4, together with the atoms to which they are bound, may form a ring; and for compounds of formula (II) one of R1a, R1b, R3 and R4 is a group comprising a linker attached to a support, and the others of R1a, R1b, R3 and R4 are independently selected optionally substituted C1-20 alkyl, optionally substituted C3-20 heterocyclyl or optionally substituted C5-20 aryl, and R1a, and R1b are additionally selected from hydrogen, or, optionally, two or more of the others of R1a, R1b, R3 and R4, together with the atoms to which they are bound, may form a ring.
表征谱图
-
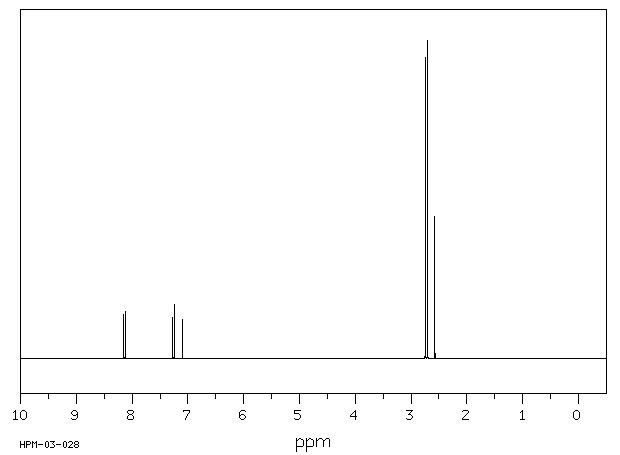
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮