2,4-二羟基-N-(2-羟乙基)苯甲酰胺 | 24207-41-8
中文名称
2,4-二羟基-N-(2-羟乙基)苯甲酰胺
中文别名
2,4-二羟基苯甲酸乙醇酰胺
英文名称
2,4-dihydroxy-N-(2-hydroxy-ethyl)benzamide
英文别名
2,4-Dihydroxy-N-(2-hydroxyethyl)benzamide
CAS
24207-41-8
化学式
C9H11NO4
mdl
MFCD00002835
分子量
197.191
InChiKey
HHSJOVPBCHTADG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:163-165 °C(lit.)
-
沸点:547.1±29.0 °C(Predicted)
-
密度:1.401±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下稳定,应避免接触强氧化剂。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:89.8
-
氢给体数:4
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2924299090
-
安全说明:S26,S37/39
制备方法与用途
用途:用于制备重氮偶合剂、硫化剂和感光剂等。
反应信息
-
作为反应物:描述:2,4-二羟基-N-(2-羟乙基)苯甲酰胺 生成 2,3-dihydro-7-hydroxy-3-(2'-hydroxyethyl)-4H-1,3-benzoxazin-4-one参考文献:名称:Benzoxazinone and benzothiazinone derivatives having cardiovascular摘要:化合物的式子为I ##STR1## 其中R代表氢,C.sub.1-C.sub.6烷基,C.sub.5-C.sub.7环烷基,亚甲二氧基或苯基,其可以被一个或两个基团独立地选择从羟基,卤素,硝基,C.sub.1-C.sub.6烷基或C.sub.1-C.sub.6烷氧基取代;R.sub.1和R.sub.2独立地代表氢,COOR.sub.3,--CONR.sub.4 R.sub.5,##STR2##--OCONR.sub.4 R.sub.5,--OCOR.sub.3,--NR.sub.4 R.sub.5,--OCOOR.sub.6,--NR.sub.3 COR.sub.7,--NR.sub.CONR.sub.4 R.sub.5,--N.dbd.CH--NR.sub.4 R.sub.5,NO.sub.2,CN,OH,SR.sub.3,其中R.sub.3为氢或C.sub.1-C.sub.6烷基,R.sub.4和R.sub.5独立地为氢或C.sub.1-C.sub.6烷基,R.sub.6为C.sub.1-C.sub.6烷基,而R.sub.7为氢,C.sub.1-C.sub.6烷基或C.sub.1-C.sub.6烷氧基,其中R.sub.1和R.sub.2不能同时为氢;X为氧或硫;Y表示C.sub.2-C.sub.6烷基链或C.sub.5-C.sub.7环烷基团;提供其药学上可接受的盐,并可用于制备药物组合物,用于治疗心血管疾病。公开号:US05480882A1
-
作为产物:描述:2,4-二羟基苯甲酸甲酯 、 alkaline earth salt of/the/ methylsulfuric acid 生成 2,4-二羟基-N-(2-羟乙基)苯甲酰胺参考文献:名称:DE896453摘要:公开号:
文献信息
-
[EN] ISOINDOLIN-1-ONE DERIVATIVES<br/>[FR] DÉRIVÉS D'ISOINDOLIN-1-ONE申请人:CANCER REC TECH LTD公开号:WO2006024837A1公开(公告)日:2006-03-09A compound of formula (1) or a prodrug and/or pharmaceutically acceptable salt thereof, wherein X is selected from O, N or S; R1 is selected from hydrogen, halo, hydroxy, substituted or unsubstituted alkyl, substituted or unsubstituted hydroxyalkyl, substituted or unsubstituted alkylamine, alkoxy, substituted or unsubstituted aryl or heteroaryl, and substituted or unsubstituted aralkyl or heteroaralkyl; R2 is selected from hydrogen, halo, hydroxy, substituted or unsubstituted alkyl, substituted or unsubstituted hydroxyalkyl substituted or unsubstituted alkylamine, alkoxy, substituted or unsubstituted aryl or heteroaryl, and substituted or unsubstituted aralkyl or heteroalkyl; R3 is selected from hydrogen, halo, hydroxy, substituted or unsubstituted alloy substituted or unsubstituted hydroxyalkyl, substituted or unsubstituted alkylamine alkoxy, substituted or unsubstituted aryl or heteroaryl, and substituted or unsubstituted aralkyl or heteroalkyl; and R4-R7, is used to represent groups R4, R5, R6 and R7 which are independently selected from H, OH, alkyl, alkoxy, alkylamine, hydroxyalkyl, halo, CF3, NH2, NO2, COOH, C=0.式(1)的化合物或其前药和/或药用可接受的盐,其中X选择自O、N或S;R1选择自氢、卤素、羟基、取代或未取代的烷基、取代或未取代的羟基烷基、取代或未取代的烷基胺、烷氧基、取代或未取代的芳基或杂环芳基、取代或未取代的芳基或杂环芳基;R2选择自氢、卤素、羟基、取代或未取代的烷基、取代或未取代的羟基烷基、取代或未取代的烷基胺、烷氧基、取代或未取代的芳基或杂环芳基、取代或未取代的芳基或杂环芳基;R3选择自氢、卤素、羟基、取代或未取代的合金取代或未取代的羟基烷基、取代或未取代的烷基胺烷氧基、取代或未取代的芳基或杂环芳基、取代或未取代的芳基或杂环芳基;R4-R7,用于表示独立选择自H、OH、烷基、烷氧基、烷基胺、羟基烷基、卤素、CF3、NH2、NO2、COOH、C=0的基团R4、R5、R6和R7。
-
[EN] CATALYST SYSTEM FOR POLYMERISATION OF AN OLEFIN<br/>[FR] SYSTÈME CATALYSEUR POUR LA POLYMÉRISATION D'UNE OLÉFINE申请人:SAUDI BASIC IND CORP公开号:WO2015022298A1公开(公告)日:2015-02-19The present invention relates to a catalyst system comprising a procatalyst, a co-catalyst and an external electron donor, wherein the external electron donor comprises a compound having the structure according to Formula I: Si (L)n (OR11)4-n (Formula I), wherein, Si is a silicon atom with valency 4+; O is an oxygen atom with valency 2- and O is bonded to Si via the silicon-oxygen bond; n is 1, 2, 3 or 4; R11 is a selected from the group consisting of linear, branched and cyclic alkyl having at most 20 carbon atoms and aromatic substituted and unsubstituted hydrocarbyl having 6 to 20 carbon atoms; L is a group represented by (Formula II), wherein, L is bonded to the silicon atom via the nitrogen-silicon bond; L has a single substituent on the nitrogen atom, where this single substituent is an imine carbon atom; and X and Y are independently selected from the group consisting of a hydrogen atom; a heteroatom selected from group 13, 14, 15, 16 or 17 of the IUPAC Periodic Table of the Elements; a linear, branched and cyclic alkyl having at most 20 carbon atoms, optionally containing a heteroatom selected from group 13, 14, 15, 16 or 17 of the IUPAC Periodic Table of the Elements and an aromatic substituted and unsubstituted hydrocarbyl having 6 to 20 carbon atoms, optionally containing a heteroatom selected from group 13, 14, 15, 16 or 7 of the IUPAC Periodic Table of the Elements.本发明涉及一种催化剂体系,包括原催化剂、协同催化剂和外部电子供体,其中外部电子供体包括具有以下结构的化合物:Si(L)n(OR11)4-n(式I),其中,Si是具有4价的硅原子;O是具有2价的氧原子,通过硅氧键与Si相连;n为1、2、3或4;R11选自具有最多20个碳原子的线性、支链和环烷基以及具有6至20个碳原子的芳香族取代和未取代的烃基;L是由(式II)表示的一组,其中,L通过氮硅键与硅原子相连;L在氮原子上具有单个取代基,该单个取代基为亚胺碳原子;X和Y分别选自IUPAC元素周期表的13、14、15、16或17族的杂原子、最多含有20个碳原子的线性、支链和环烷基,可以包含来自IUPAC元素周期表的13、14、15、16或17族的杂原子,以及具有6至20个碳原子的芳香族取代和未取代的烃基,可以包含来自IUPAC元素周期表的13、14、15、16或17族的杂原子。
-
Isoindolin-1-One Derivatives申请人:Willems Hendrika Maria Gerarda公开号:US20080261917A1公开(公告)日:2008-10-23A compound of formula or a prodrug and/or a pharmaceutically acceptable salt thereof, wherein X is O, N or S; R 1 is hydrogen, halo, hydroxy, substituted or unsubstituted alkyl, substituted or unsubstituted hydroxyalkyl, substituted or unsubstituted alkylamine, alkoxy, substituted or unsubstituted aryl or heteroaryl, and substituted or unsubstituted aralkyl or heteroaralkyl; R 2 is hydrogen, halo, hydroxy, substituted or unsubstituted alkyl, substituted or unsubstituted hydroxyalkyl substituted or unsubstituted alkylamine, alkoxy, substituted or unsubstituted aryl or heteroaryl, and substituted or unsubstituted aralkyl or heteroalkyl; R 3 is hydrogen, halo, hydroxy, substituted or unsubstituted alloy substituted or unsubstituted hydroxyalkyl, substituted or unsubstituted alkylamine alkoxy, substituted or unsubstituted aryl or heteroaryl, and substituted or unsubstituted aralkyl or heteroalkyl; and R 4 -R 7 , is used to represent groups R 4 , R 5 , R 6 and R 7 which are H, OH, alkyl, alkoxy, alkylamine, hydroxyalkyl, halo, CF 3 , NH 2 , NO 2 , COOH, C=0.该化合物的化学式为,或其前体药物和/或其药学上可接受的盐,其中X为O、N或S;R1为氢、卤、羟基、取代或未取代的烷基、取代或未取代的羟基烷基、取代或未取代的烷基胺、烷氧基、取代或未取代的芳基或杂环芳基、取代或未取代的芳基烷基或杂环芳基烷基;R2为氢、卤、羟基、取代或未取代的烷基、取代或未取代的羟基烷基、取代或未取代的烷基胺、烷氧基、取代或未取代的芳基或杂环芳基、取代或未取代的芳基烷基或杂环芳基烷基;R3为氢、卤、羟基、取代或未取代的烷基、取代或未取代的羟基烷基、取代或未取代的烷基胺、烷氧基、取代或未取代的芳基或杂环芳基、取代或未取代的芳基烷基或杂环芳基烷基;R4-R7用于代表R4、R5、R6和R7的基团,其为H、OH、烷基、烷氧基、烷基胺、羟基烷基、卤、CF3、NH2、NO2、COOH、C=0。
-
CATALYST SYSTEM FOR POLYMERISATION OF AN OLEFIN申请人:ZUIDEVELD Martin Alexander公开号:US20160176998A1公开(公告)日:2016-06-23The present invention relates to a catalyst system comprising a procatalyst, a co-catalyst and an external electron donor, wherein the external electron donor comprises a compound having the structure according to Formula I: Si(L) n (OR 11 ) 4-n (Formula I), wherein, Si is a silicon atom with valency 4+; O is an oxygen atom with valency 2− and O is bonded to Si via the silicon-oxygen bond; n is 1, 2, 3 or 4; R 11 is a selected from the group consisting of linear, branched and cyclic alkyl having at most 20 carbon atoms and aromatic substituted and unsubstituted hydrocarbyl having 6 to 20 carbon atoms; L is a group represented by (Formula II), wherein, L is bonded to the silicon atom via the nitrogen-silicon bond; L has a single substituent on the nitrogen atom, where this single substituent is an imine carbon atom; and X and Y are independently selected from the group consisting of a hydrogen atom; a heteroatom selected from group 13, 14, 15, 16 or 17 of the IUPAC Periodic Table of the Elements; a linear, branched and cyclic alkyl having at most 20 carbon atoms, optionally containing a heteroatom selected from group 13, 14, 15, 16 or 17 of the IUPAC Periodic Table of the Elements and an aromatic substituted and unsubstituted hydrocarbyl having 6 to 20 carbon atoms, optionally containing a heteroatom selected from group 13, 14, 15, 16 or 7 of the IUPAC Periodic Table of the Elements.本发明涉及一种催化剂体系,包括原催化剂、协同催化剂和外部电子给体,其中,外部电子给体包括具有以下结构的化合物,即式I:Si(L)n(OR11)4-n(式I),其中,Si是价态为4+的硅原子;O是价态为2-的氧原子,通过硅氧键与Si相连;n为1、2、3或4;R11是从线性、支链和环烷烃中选择的,其最多含有20个碳原子,并且是芳香取代和未取代的烃基,其碳原子数为6至20个;L是由式II表示的基团,其中,L通过氮硅键与硅原子连接;L在氮原子上有一个单取代基团,该单取代基团是亚胺碳原子;X和Y独立地选择自元素周期表中第13、14、15、16或17族的杂原子、最多含有20个碳原子的线性、支链和环烷烃,可选含有元素周期表中第13、14、15、16或17族的杂原子和含有6至20个碳原子的芳香取代和未取代的烃基,可选含有元素周期表中第13、14、15、16或17族的杂原子。
-
Diazotype compositions and materials申请人:Andrews Paper and Chemical Co., Inc.公开号:EP0122523A2公开(公告)日:1984-10-24The use of soluble methylol compounds from primary or secondary amines or ammonia with formaldehyde in diazotypes improves solution compatibility and prevents coupler migration upon storage of diazotype materials. The methylol compounds may be reacted with couplers separately or added to the coating preparations.
表征谱图
-
氢谱1HNMR
-
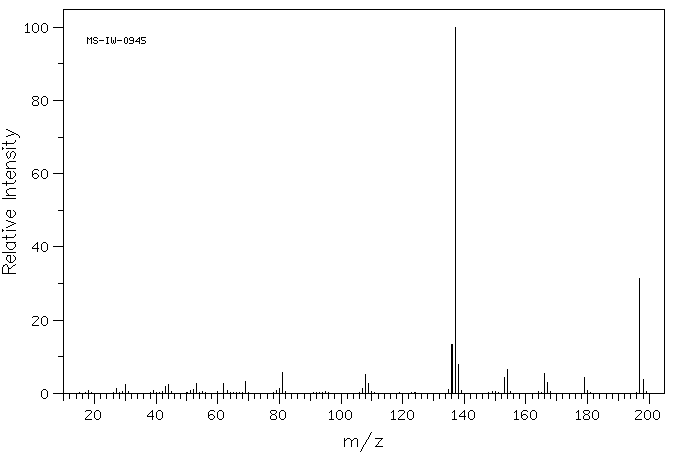
质谱MS
-
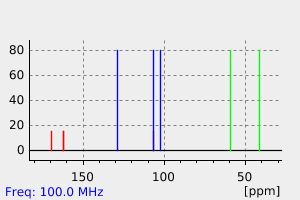
碳谱13CNMR
-
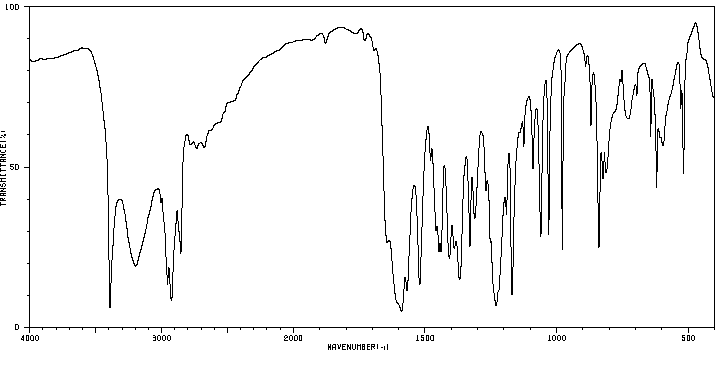
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫