2-(苯甲酰基氨基)-6-氯苯甲酸 | 19407-43-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:19
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-6-氯苯甲酸 2-chloro-6-aminobenzoic acid 2148-56-3 C7H6ClNO2 171.583 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-6-氯苯甲酸 2-chloro-6-aminobenzoic acid 2148-56-3 C7H6ClNO2 171.583
反应信息
-
作为反应物:描述:参考文献:名称:Uhle, Journal of the American Chemical Society, 1949, vol. 71, p. 761,763摘要:DOI:
-
作为产物:参考文献:名称:Uhle, Journal of the American Chemical Society, 1949, vol. 71, p. 761,763摘要:DOI:
文献信息
-
Changes in the Activity and Selectivity of Herbicides by Selective Fluorine Substitution, Taking Bentranil and Classic® Analogues as Examples作者:Gerhard Hamprecht、Bruno Würzer、Matthias WitschelDOI:10.2533/000942904777678226日期:——
The introduction of fluorine atoms into 2-phenyl-4H-3,1-benzoxazin-4-one (bentranil) led to sweeping changes in its herbicidal properties in some cases, and 5-fluoro-2-phenyl-4H-3,1-benzoxazin-4-one ('fluorobentranil') was found to be the most active compound. It can be prepared from 2-amino-6-fluoro-benzoic acid or by direct halogen exchange of 5-chloro-2-phenyl-4H-3,1-benzoxazin-4-one. The latter reaction was investigated on a pilot scale, including a high-temperature (350 °C) potassium fluoride halogen exchange without solvent. When sulfolane was used as a solvent, a side reaction at 220 °C – partial decomposition to a diphenylether – could be prevented by addition of a small amount of a radical scavenger. Other intermediates with a pseudohalogen substitution were obtained by side-chain chlorination of suitable methylsulfanyl benzoic acid precursors and halogen exchange. 'Fluorobentranil' shows good broad-leaf activity and selectivity on rice, cereals and maize. In a second case study, the fluoro-substituted anthranilic acids mentioned above were also found to be appropriate for synthesizing herbicidal sulfonylurea (SU) compounds via Meerwein reaction of their aniline function. Methyl 2-[([(4chloro-6-methoxy-2-pyrimidinyl)-amino]carbonyl}amino)sulfonyl]-6-fluorobenzoate is an example of a SU that is compatible with maize, whereas the unsubstituted Classic® analogue is not selective.
将氟原子引入2-苯基-4H-3,1-苯并噁嗪-4-酮(苯噁嗪)中,在某些情况下会对其除草性能产生巨大影响,5-氟-2-苯基-4H-3,1-苯并噁嗪-4-酮('氟苯噁嗪')被发现是最活性的化合物。它可以由2-氨基-6-氟苯甲酸制备,或通过直接卤素交换5-氯-2-苯基-4H-3,1-苯并噁嗪-4-酮来制备。后一种反应在试验规模上进行了研究,包括高温(350°C)下无溶剂的氟化钾卤素交换。当作为溶剂使用戊磺醚时,220°C时可能发生的一个副反应——部分分解为二苯醚——可以通过添加少量自由基清除剂来防止。通过侧链氯化适当的甲基磺基苯甲酸前体和卤素交换获得了其他具有伪卤素取代的中间体。'氟苯噁嗪'在水稻、谷物和玉米上表现出良好的广谱活性和选择性。在第二个案例研究中,上述氟代蒽醌酸也被发现适合用于通过其苯胺功能的Meerwein反应合成除草磺酰脲(SU)化合物。2-[(4-氯-6-甲氧基-2-嘧啶基)氨基]羰基]氨基]磺酰]-6-氟苯甲酸甲酯是与玉米兼容的SU的一个例子,而未取代的Classic®类似物则不具有选择性。 -
Verfahren zur Herstellung substituierter 2-Phenyl-4H-3,1-benzoxazin-4-one申请人:BASF Aktiengesellschaft公开号:EP0199276A2公开(公告)日:1986-10-29Verfahren zur Herstellung von 2-Phenyl-4H-3,1-benzoxazin-4-onen der allgemeinen Formel I worin R' und R2 Wasserstoff oder Halogen, R' ferner Methyl oder Methoxy, R2 ferner Halogenalkyl, Halogenalkoxy, Halogenalkylmercapto oder Halogenalkylsulfonyl mit jeweils 1 bis 3 Kohlenstoffatomen bedeutet, durch Umsetzung einer entsprechenden Anthranilsäure mit einem entsprechenden Benzoylhalogenid in Gegenwart einer Base - (Acylierung) und Ringschluß durch Wasserentzug - (Cyclisierung), in einem mit wäßriger Alkalilauge nicht mischbaren Suspensionsmittel, in Gegenwart eines Phasentransferkatalysators.
-
——作者:HAMPRECHT G.、 ROHR W.、 VARWIG J.DOI:——日期:——
-
US4187317A申请人:——公开号:US4187317A公开(公告)日:1980-02-05
-
US4230727A申请人:——公开号:US4230727A公开(公告)日:1980-10-28
表征谱图
-
氢谱1HNMR
-
质谱MS
-
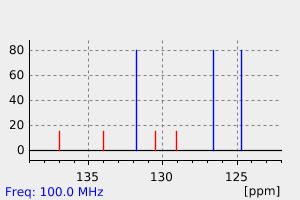
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息