N,N'-Ethylenebis(N-ethylbenzamide) | 82126-36-1
中文名称
——
中文别名
——
英文名称
N,N'-Ethylenebis(N-ethylbenzamide)
英文别名
N-[2-[benzoyl(ethyl)amino]ethyl]-N-ethylbenzamide
CAS
82126-36-1
化学式
C20H24N2O2
mdl
——
分子量
324.423
InChiKey
YHRJLFAAPKRCIK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:24
-
可旋转键数:7
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:40.6
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为产物:描述:参考文献:名称:含硅衍生物的合成:对称二胺的非对称衍生摘要:描述了通过使用环状硅氮化合物作为中间体将对称的二胺转化为单酰胺。DOI:10.1016/s0040-4039(00)86999-3
文献信息
-
Selective Monoacylation of Symmetrical Diamines via Prior Complexation with Boron作者:Zhongxing Zhang、Zhiwei Yin、Nicholas A. Meanwell、John F. Kadow、Tao WangDOI:10.1021/ol0300773日期:2003.9.1[reaction: see text] Pretreatment of a symmetrical primary or secondary diamine with 9-BBN prior to the addition of an acyl chloride significantly suppressed undesired diacylation, and the product of monoacylation predominated. The reactive preference is interpreted as the result of a selective deactivation of one nitrogen atom of the diamine by 9-BBN.[反应:见正文]在添加酰氯之前,用9-BBN预处理对称的伯或仲二胺会显着抑制不希望的二酰化作用,并且单酰化产物占主导地位。反应性偏爱被解释为二胺的一个氮原子被9-BBN选择性失活的结果。
-
Imidazole-Catalyzed Monoacylation of Symmetrical Diamines作者:Sanjeev K. Verma、B. N. Acharya、M. P. KaushikDOI:10.1021/ol101604q日期:2010.10.1An imidazole-catalyzed protocol for monoacylation of symmetrical diamines has been developed. The protocol gave selective monoacylation of aliphatic (cyclic and acyclic) primary and secondary diamines. In the reaction, imidazole acts as both catalyst and a leaving group. Different monoacylated piperazines and other diamines were synthesized at room temperature in an ethanol/water solvent system.
-
SCHWARTZ, E.;SHANZER, A., TETRAHEDRON LETT., 1982, 23, N 9, 979-982作者:SCHWARTZ, E.、SHANZER, A.DOI:——日期:——
-
US4386053A申请人:——公开号:US4386053A公开(公告)日:1983-05-31
表征谱图
-
氢谱1HNMR
-
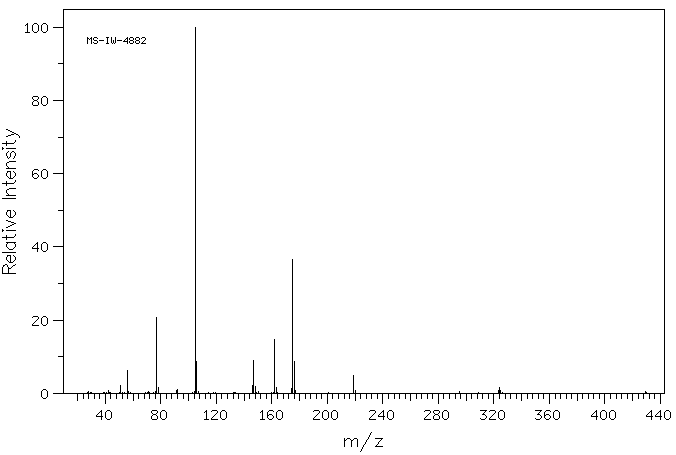
质谱MS
-
碳谱13CNMR
-
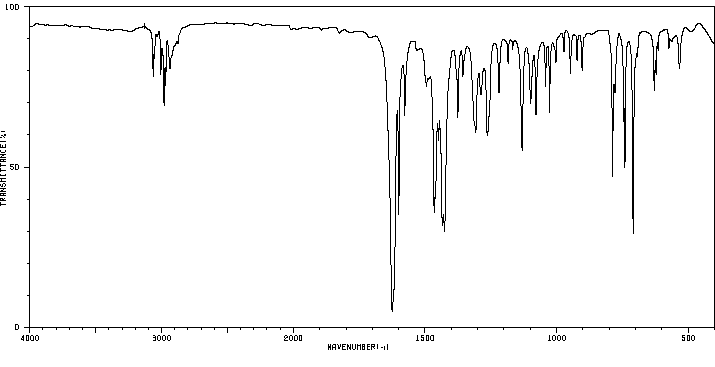
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫