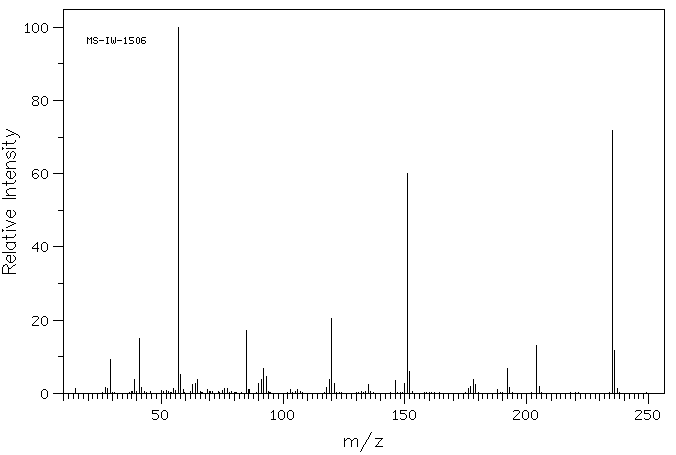
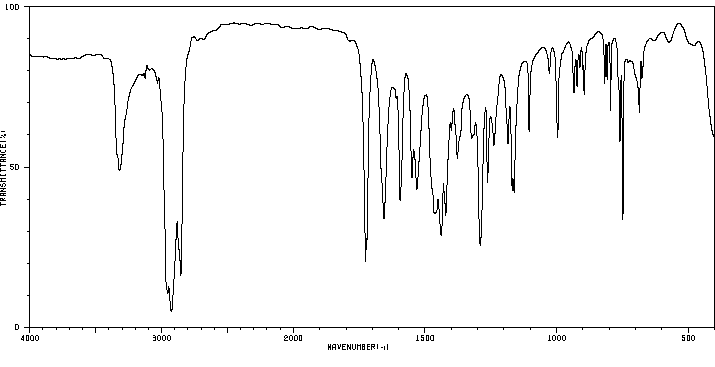
2-[(2,2-二甲基-1-氧代丙基)氨基]苯甲酸甲酯 | 84540-62-5
中文名称
2-[(2,2-二甲基-1-氧代丙基)氨基]苯甲酸甲酯
中文别名
——
英文名称
methyl 2-pivalamidobenzoate
英文别名
Methyl 2-[(2,2-dimethyl-1-oxopropyl)amino]benzoate;methyl 2-(2,2-dimethylpropanoylamino)benzoate
CAS
84540-62-5
化学式
C13H17NO3
mdl
MFCD00008841
分子量
235.283
InChiKey
TWDCYCSGTBPHFH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:17
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.384
-
拓扑面积:55.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-[(2,2-二甲基-1-氧代丙基)氨基]苯甲酸 2-pivalamidobenzoic acid 101724-84-9 C12H15NO3 221.256 邻氨基苯甲酸甲酯 2-carbomethoxyaniline 134-20-3 C8H9NO2 151.165 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-neopentylaminobenzyl alcohol 137782-23-1 C12H19NO 193.289
反应信息
-
作为反应物:描述:2-[(2,2-二甲基-1-氧代丙基)氨基]苯甲酸甲酯 在 tetraphosphorus decasulfide 作用下, 生成 t-butyl-2 benzothiazine-3,1 thione-4参考文献:名称:Legrand,L., Bulletin de la Societe Chimique de France, 1960, p. 337 - 343摘要:DOI:
-
作为产物:描述:苯甲醯亞胺酸 在 potassium carbonate 、 silver(l) oxide 作用下, 以 乙腈 为溶剂, 反应 13.0h, 生成 2-[(2,2-二甲基-1-氧代丙基)氨基]苯甲酸甲酯参考文献:名称:银催化邻氨基苯甲酸和羧酸实际合成 N-酰基邻氨基苯甲酸摘要:通过银催化从容易获得的邻氨基苯甲酸和羧酸生成亚氨基乙烯酮,实现了有价值的N-酰基邻氨基苯甲酸的实际合成。多种羧酸,包括空间要求高的脂肪族羧酸、芳香族羧酸、丙烯酸和氨基酸,都适合该反应。此外,该方法还可用于修饰药物分子和天然产物,例如布洛芬、丙磺舒和乙酰甘氨酸。DOI:10.1021/acs.joc.3c02586
文献信息
-
Site-Selective C–H Functionalization of (Hetero)Arenes via Transient, Non-symmetric Iodanes作者:Stacy C. Fosu、Chido M. Hambira、Andrew D. Chen、James R. Fuchs、David A. NagibDOI:10.1016/j.chempr.2018.11.007日期:2019.2A strategy for C–H functionalization of arenes and heteroarenes has been developed to allow site-selective incorporation of various anions, including Cl, Br, OMs, OTs, and OTf. This approach is enabled by in situ generation of reactive, non-symmetric iodanes by combining anions and bench-stable PhI(OAc)2. The utility of this mechanism is demonstrated via para-selective chlorination of medicinally relevant
-
Asymmetric Synthesis of N–N Axially Chiral Compounds by Phase-Transfer-Catalyzed Alkylations作者:Ming Pan、Ying-Bo Shao、Qun Zhao、Xin LiDOI:10.1021/acs.orglett.1c04028日期:2022.1.14N–N axially chiral skeletons are significant structural motifs in natural products, pharmaceuticals, and functional materials. Herein we disclose a method for the asymmetric synthesis of N–N axially chiral compounds by phase-transfer catalysis. A wide range of N–N axially chiral quinazolinone derivatives were prepared in high yields with excellent stereoselectivities. Furthermore, the synthetic utility
-
Triptycenyl Sulfide: A Practical and Active Catalyst for Electrophilic Aromatic Halogenation Using <i>N</i>-Halosuccinimides作者:Yuji Nishii、Mitsuhiro Ikeda、Yoshihiro Hayashi、Susumu Kawauchi、Masahiro MiuraDOI:10.1021/jacs.9b12672日期:2020.1.22A Lewis base catalyst Trip-SMe (Trip = triptycenyl) for electrophilic aromatic halogenation using N-halosuccinimides (NXS) is introduced. In the presence of an appropriate activator (as a non-coordinating-anion source), a series of unactivated aromatic compounds were halogenated at ambient temperature using NXS. This catalytic system was applicable to transformations that are currently unachievable介绍了一种用于使用 N-卤代琥珀酰亚胺 (NXS) 进行亲电芳香卤化的路易斯碱催化剂 Trip-SMe(Trip = triptycenyl)。在合适的活化剂(作为非配位阴离子源)存在下,一系列未活化的芳香族化合物在环境温度下使用 NXS 进行卤化。该催化体系适用于目前除使用 Br2 或 Cl2 之外无法实现的转化:例如萘的多卤化、BINOL 的区域选择性溴化等。 对照实验表明,三烯基取代基对催化活性起着至关重要的作用,和动力学实验暗示锍盐 [Trip-S(Me)Br][SbF6] 作为活性物质的存在。与简单的二烷基硫醚相比,Trip-SMe 在卤络合物中表现出显着的电荷分离离子对特征,其结构信息是通过单晶 X 射线分析获得的。一项初步的计算研究表明,三烯基官能团的 π 系统是巩固亲电性增强的关键基序。
-
Ligand Promoted Olefination of Anilides for Indirectly Introducing Fluorinated Functional Groups via Palladium Catalyst作者:Dongjie Wang、Xu Xu、Jingyu Zhang、Yingsheng ZhaoDOI:10.1021/acs.joc.0c02701日期:2021.2.5We report a palladium-catalyzed, ligand promoted, C–H fluorine-containing olefination of anilides with 4-bromo-3,3,4,4-tetrafluorobutene as the fluorinated reagent, which has a potential transformation into other compounds due to its -CF2CF2Br functional group. -CF2CF2H was obtained by using the mild reducing agent sodium borohydride. Bioactive compounds such as aminoglutethimide derivative and propham
-
Synthesis and Structure-Activity Relationships of N-Substituted 2-((2-Imidazolylsulfinyl)methyl)anilines as a New Class of Gastric H+/K+-ATPase Inhibitors.作者:Tomio YAMAKAWA、Hitoshi MATSUKURA、Yutaka NOMURA、Mitsuko YOSHIOKA、Mitsuo MASAKI、Hideki IGATA、Susumu OKABEDOI:10.1248/cpb.39.1746日期:——activity against gastric H+/K(+)-ATPase prepared from rabbit stomach and gastric acid secretions in Heidenhain pouch dogs. Monoalkyl substituents on the nitrogen atom of the aniline moiety markedly inhibited the enzyme activity to the same degree as omeprazole, a representative H+/K(+)-ATPase inhibitor. Most of these compounds, administered at 3 mg/kg i.v. inhibited histamine-stimulated gastric acid secretion
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫