5β-androst-3-en-17β-yl acetate | 6197-83-7
分子结构分类
中文名称
——
中文别名
——
英文名称
5β-androst-3-en-17β-yl acetate
英文别名
17β-acetoxy-5β-androst-3-ene;acetic acid-(5β-androst-3-en-17β-yl ester);Essigsaeure-(5β-androst-3-en-17β-ylester);[(5R,8R,9S,10S,13S,14S,17S)-10,13-dimethyl-2,5,6,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl] acetate
CAS
6197-83-7
化学式
C21H32O2
mdl
——
分子量
316.484
InChiKey
CLULEPXOEJTROX-BPSSIEEOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:142-143 °C
-
沸点:388.9±42.0 °C(Predicted)
-
密度:1.05±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:23
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:STEROIDS。CCV。响一个修改过的激素模拟。I.敲响烯烃。摘要:DOI:10.1021/jm00338a016
-
作为产物:描述:参考文献:名称:Configuration of Steroid Bromoketones. II. 4β-Bromotestane-17β-ol-3-one Acetate and 2β-Bromocholestane-3-one摘要:DOI:10.1021/ja01103a055
文献信息
-
Regioselective enzymatic acylation of vicinal diols of steroids作者:M. Manuel Cruz Silva、Sergio Riva、M. Luisa Sá e MeloDOI:10.1016/j.tet.2005.01.104日期:2005.3Monoacylated derivatives of a complete set of 2,3- and 3,4-vicinal diols of steroids were prepared by regioselective lipase-catalysed transesterification reactions. The enzymes displayed different selectivities towards the vicinal diols depending on the configuration of the hydroxyl groups.
-
Ultrasound assisted zinc reactions in synthesis 1. Efficient reduction of enones作者:J.A.R. Salvador、M.L.Sa´ e Melo、A.S. Campos NevesDOI:10.1016/s0040-4039(00)60587-7日期:1993.1Zinc reduction of α, β-unsaturated ketones in acetic acid has been efficiently accomplished under sonochemical conditions. Different α-enone systems give two kinds of products: olefins and allylic alcohols. Regio- and stereoselective are reported.
-
The Stereochemistry of an Elimination Reaction accompanying the Hydroboration of a Steroidal Allylic Alcohol†作者:James R. Hanson、Mansur D. Liman、Cavit UyanikDOI:10.1039/a708181b日期:——Deuterium labelling studies have shown that the facile elimination of the 5β-hydroxy group observed in the course of hydroboration of a 5β-hydroxyandrost-3-ene may involve a trans diaxial borane-borinate elimination coupled with a syn transfer of hydrogen from the borinate.
-
McKenna et al., Journal of the Chemical Society, 1959, p. 2502,2507作者:McKenna et al.DOI:——日期:——
-
Crastes de Paulet,A.; Bascoul,J., Bulletin de la Societe Chimique de France, 1966, p. 939 - 944作者:Crastes de Paulet,A.、Bascoul,J.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
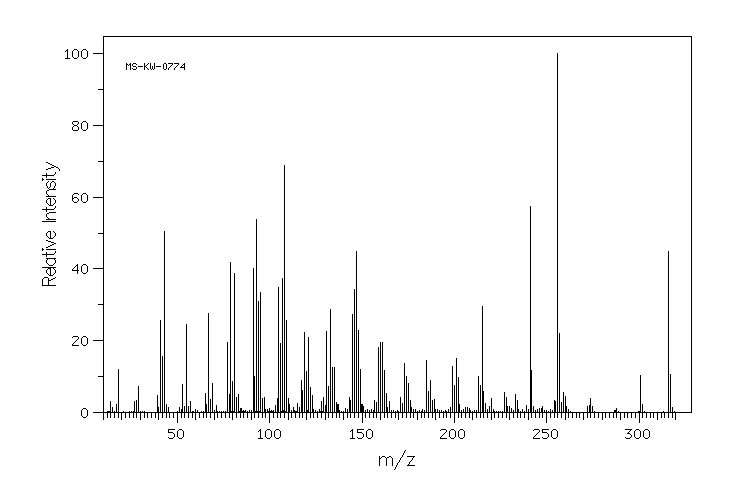
质谱MS
-
碳谱13CNMR
-
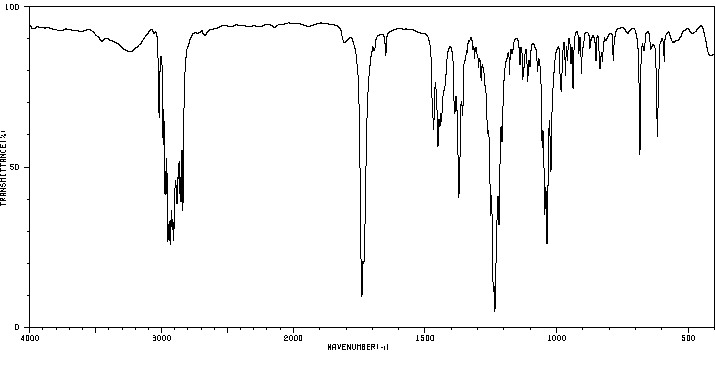
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B