苯并(a)晕苯 | 190-70-5
中文名称
苯并(a)晕苯
中文别名
——
英文名称
benzo[a]coronene
CAS
190-70-5
化学式
C28H14
mdl
——
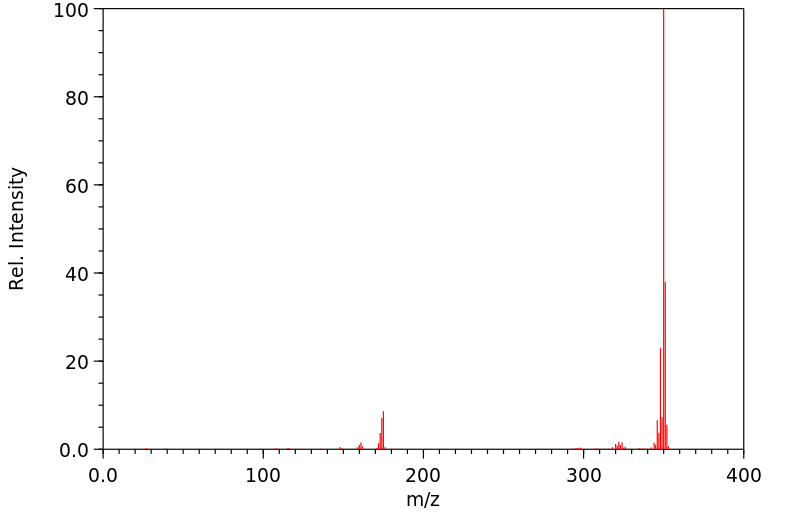
分子量
350.419
InChiKey
NQSLOOOUQZYGEB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:292-294.5 °C(Solv: xylene (1330-20-7))
-
沸点:604.8±22.0 °C(Predicted)
-
密度:1.467±0.06 g/cm3(Predicted)
-
保留指数:655.84;656.34
计算性质
-
辛醇/水分配系数(LogP):8
-
重原子数:28
-
可旋转键数:0
-
环数:8.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2914399090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:碳氢化合物的高温稳定性摘要:DOI:10.1021/j100263a027
-
作为产物:参考文献:名称:不对称和对称的成核作用—III:苯甲酮摘要:通过二酐(III)从1:2–4:5–8:9-三苯并py(II)中获得1:2–5:6-二苯并co(IV)。1:2–4:5-二苯并py(V)与马来酸酐冷凝两次。所得的二酐(VI)在脱羧后得到1:2-苯并二氢呋喃(VII)。通过将锌粉从醌(VIII)熔融而获得1∶12-邻苯二甲酰((X)。从三亚苯基和per到苯并氢醌的环化作用表明在苯二胺的电子精细结构内存在三亚苯基配合物。DOI:10.1016/0040-4020(59)80017-x
文献信息
-
Short and Efficient Synthesis of Coronene Derivatives via Ruthenium-Catalyzed Benzannulation Protocol作者:Hung-Chin Shen、Jhih-Meng Tang、Hsu-Kai Chang、Chia-Wei Yang、Rai-Shung LiuDOI:10.1021/jo0512599日期:2005.11.1TpRuPPh3(CH3CN)2PF6 (3 mol %) was very active in catalytic benzannulation of 1-phenyl-2-ethynylbenzenes in dichloroethane (60 °C, 36 h) to afford phenanthrene in 95% yield. This method is applicable to the synthesis of various polycyclic aromatic hydrocarbons via two- and four-fold benzannulations, including various substituted coronene derivatives (53−86% yields) using this catalyst at a moderate
-
Bunte, Reinhard; Gundermann, Karl-Dietrich; Leitich, Johannes, Chemische Berichte, 1986, vol. 119, # 12, p. 3521 - 3527作者:Bunte, Reinhard、Gundermann, Karl-Dietrich、Leitich, Johannes、Polansky, Oskar E.、Zander, MaximilianDOI:——日期:——
-
Photodehydrocyclizations in stilbene-like compounds. IX. 1,2-Phenyl shifts in the cyclization of 1-phenylpentahelicenes作者:A. H. A. Tinnemans、W. H. LaarhovenDOI:10.1021/ja00821a042日期:1974.7
-
Zander,M.; Franke,W.H., Chemische Berichte, 1966, vol. 99, p. 1275 - 1278作者:Zander,M.、Franke,W.H.DOI:——日期:——
-
314. 1 : 2-Benzocoronene and naphtho(2′ : 3′-1 : 2)coronene作者:E. Clar、M. ZanderDOI:10.1039/jr9580001577日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
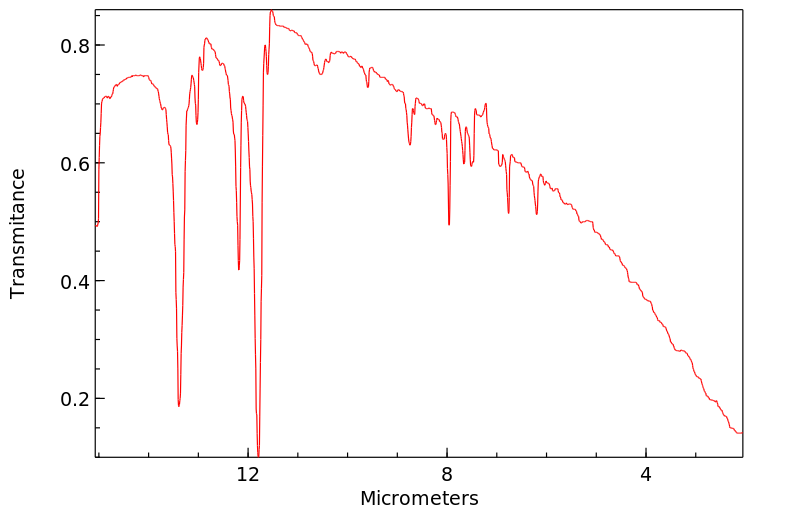
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1,2-二(1-芘基)环丁烷
顺式-1,2-二(1-芘基)环丁烷
顺式-(-)-苯并(a)芘-7,8-二醇-9,10-环氧化物
雄甾烷
还原黑29
还原黄4
还原金橙G
还原绿2
还原绿1
还原紫3B
还原紫 10
还原深蓝BO
还原橙4
还原橙2
还原兰黑BBN
还原亮橙IRK
试剂N1,N1,N3,N3,N6,N6,N8,N8-Octakis(4-methoxyphenyl)-1,3,6,8-pyrenetetramine
蒽酮紫79
蒽缔蒽酮
蒽并(1,2,3,4-ghi)苝
蒽嵌蒽
蒽[9,1,2-cde]苯并[rst]戊芬
萘并[2'.8',2.4]晕苯
萘并[2',1',8',7':4,10,5]蒽并[1,9,8-abcd]晕苯
萘并[1,8-gh:4,5-g'h']二喹啉
萘并(8,1,2-bcd)苝
萘并(2,3-a)晕苯
萘并(2,1,8-qra)萘并萘-7 12-二酮
萘并(1,2,3-mno)醋菲烯
萘[2,3-a]芘
菲并[1,10,9,8-opqra]苝
茚并(1,2,3-cd)芘
苯胺,2-氯-3-(苯基甲氧基)-
苯并[xyz]庚芬
苯并[wx]萘并[2,1,8,7-hijk]庚省
苯并[rst]菲并[1,10,9-cde]戊芬
苯并[rst]戊酚-5-甲醛
苯并[pqr]四苯-5-基甲酸根
苯并[pqr]四苯-11-基甲酸根
苯并[pqr]二萘并[8,1,2-bcd:2',1',8'-lmn]苝
苯并[p]萘并[1,8,7-ghi]屈
苯并[l]芘-8-醇
苯并[ghi]苝
苯并[e]芘
苯并[b]芘-6-基甲醇
苯并[b]芘-6,12-二酮
苯并[b]芘-3,6-二酮
苯并[b]芘-1,6-二酮
苯并[a]芘-9,10-环氧化物
苯并[a]芘-7-醇