4-硝基苯基壬醚 | 86702-46-7
中文名称
4-硝基苯基壬醚
中文别名
4-硝基苯基壬基醚
英文名称
4-nitrophenyl nonyl ether
英文别名
4-Nonyloxynitrobenzene;NB-9;(4-nitro-phenyl)-nonyl ether;(4-Nitro-phenyl)-nonyl-aether;4-Nitro-1-nonyloxy-benzol;p-Nitrophenyl nonyl ether;1-nitro-4-nonoxybenzene
CAS
86702-46-7
化学式
C15H23NO3
mdl
MFCD00043612
分子量
265.353
InChiKey
JLSCIQUWVGKSDH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:188-189 °C/7 mmHg (lit.)
-
密度:1.029 g/mL at 25 °C (lit.)
-
闪点:>230 °F
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:19
-
可旋转键数:9
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:3
SDS
Version 1.2
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 4-NITROPHENYL NONYL ETHER, 98%
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
4-NITROPHENYL NONYL ETHER, 98% 86702-46-7 None None
Formula C15H23NO3
Molecular Weight 265,3600 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact, immediately flush eyes with copious amounts
of water for at least 15 minutes.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Carbon dioxide, dry chemical powder, or appropriate
foam.
ALDRICH www.molbase.com
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid inhalation. Do not get in
eyes, on skin, on clothing. Avoid prolonged or repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed. Store in a cool dry
place.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling. Wash contaminated clothing before
reuse.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Respiratory protection is not required. Where
protection is desired, use multi-purpose combination (US) or type
ABEK (EN 14387) respirator cartridges.
Hand Protection: Rubber gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
pH N/A
BP/BP Range 188,000. - 189,000 °7,000 mmHg
C.
MP/MP Range N/A
Flash Point 113,000 °C Method: closed cup
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
SG/Density 1,0290 g/cm3
ALDRICH www.molbase.com
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Materials to Avoid: Strong oxidizing agents, Strong bases.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides.
11 - Toxicological Information
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Eye Contact: May cause eye irritation.
Multiple Routes: May be harmful by inhalation, ingestion, or
skin absorption.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
ALDRICH www.molbase.com
Caution: Substance not yet fully tested (EU).
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 4-NITROPHENYL NONYL ETHER, 98%
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
4-NITROPHENYL NONYL ETHER, 98% 86702-46-7 None None
Formula C15H23NO3
Molecular Weight 265,3600 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact, immediately flush eyes with copious amounts
of water for at least 15 minutes.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Carbon dioxide, dry chemical powder, or appropriate
foam.
ALDRICH www.molbase.com
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid inhalation. Do not get in
eyes, on skin, on clothing. Avoid prolonged or repeated exposure.
STORAGE
Conditions of Storage: Keep tightly closed. Store in a cool dry
place.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling. Wash contaminated clothing before
reuse.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Respiratory protection is not required. Where
protection is desired, use multi-purpose combination (US) or type
ABEK (EN 14387) respirator cartridges.
Hand Protection: Rubber gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
pH N/A
BP/BP Range 188,000. - 189,000 °7,000 mmHg
C.
MP/MP Range N/A
Flash Point 113,000 °C Method: closed cup
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
SG/Density 1,0290 g/cm3
ALDRICH www.molbase.com
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Materials to Avoid: Strong oxidizing agents, Strong bases.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides.
11 - Toxicological Information
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Eye Contact: May cause eye irritation.
Multiple Routes: May be harmful by inhalation, ingestion, or
skin absorption.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
ALDRICH www.molbase.com
Caution: Substance not yet fully tested (EU).
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:三组分反应喹啉:合成与光物理研究摘要:由三组分描述了五种喹啉8-辛氧基-4- [4-(辛氧基氧基)苯基]喹啉和6-烷氧基-2-(4-烷氧基苯基)-4-[(4-辛氧基氧基)芳基]喹啉的合成。路易斯酸FeCl3和Yb(OTf)3介导的偶联反应。4-n-辛基氧基苯甲醛,茴香醛,4-n-辛基氧基苯胺对茴香胺和1-乙炔基-4-庚基氧基苯,1-乙炔基-4-辛基苯氧基和2-乙炔基-6-庚基氧基萘是该方案中的试剂。Yb3 +催化剂比Fe3 +产生更高的喹啉收率。偏光光学显微镜(POM)显示,喹啉都不是液晶,甚至各向异性更强。极性溶剂中喹啉之一的UV-Vis测量显示,在280和350 nm处有两个吸收带,与π,π*和n,π*跃迁有关。观察到较低的能量吸收带没有变化(e < 104 mol L-1 cm-1)与n,π*跃迁有关。对一种喹啉的激光闪光光解研究涉及在乙醇中寿命为2.6 µs的450 nm主瞬变带,该瞬变带在氧气存在下被完全淬灭。将该瞬DOI:10.5935/0103-5053.20150011
-
作为产物:参考文献:名称:CRISTEA, M. F.;JACOB, N.;JACOB, M.;HODOSAN, F. P.摘要:DOI:
文献信息
-
The effect of position of (S)-2-octyloxy tail on the formation of frustrated blue phase and antiferroelectric phase in Schiff base liquid crystals作者:Chiung-Cheng Huang、Ching-Chung Hsu、Li-Wen Chen、Yu-Lun ChengDOI:10.1039/c4sm01829j日期:——
Chiral salicylaldimine-based liquid crystals in which the (
S )-2-octyloxyl tail close to or far from the salicylaldimine core possesses a frustrated blue phase and an antiferroelectric SmC*A phase. -
Liquid Crystalline Properties of Dimers Having o-, m- and p- Positional Molecular Structures作者:Joo-Hoon Park、Ok-Byung Choi、Hwan-Myung Lee、Jin-Young Lee、Sung-Jo Kim、Eun-Hee Cha、Dong-Hyun Kim、B. Ramaraj、Bong-Keun So、Kyung-Hwan Kim、Soo-Min Lee、Kuk-Ro YoonDOI:10.5012/bkcs.2012.33.5.1647日期:2012.5.20With the objective to design and synthesis of Schiff's base symmetrical liquid crystal dimmers and to study the effect of molecular structure variation (o-ortho, m-meta, p-para) and change in alkoxy terminal chain length on mesomorphic properties of liquid crystals, We have synthesized Schiff base dimers from dialdehyde derivative containing 2-hydroxy-1,3-dioxypropylene as short spacer with aniline以设计和合成希夫碱对称液晶二聚体为目的,研究分子结构变化(邻位、间位、对位)和烷氧基末端链长变化对液晶介晶性质的影响,我们从含有 2-羟基-1,3-二氧丙烯作为短间隔物的二醛衍生物与具有不同末端烷氧基链长度 (n = 5, 7, 9) 的苯胺衍生物合成了席夫碱二聚体。通过质子核磁共振( 1 H NMR)光谱和傅里叶变换红外(FT-IR)光谱表征最终产物的化学结构。所得二聚体的介晶性质和光学结构通过差示扫描量热法 (DSC) 和偏光显微镜 (POM) 进行表征。当化合物从结晶相加热时,通过在光学显微镜中观察到棒状网和扇形纹理,证实了近晶 A 相变的存在。该系列的所有二聚体,除2S5-ortho、-meta、-para外,均为热致液晶。化合物 2S9 -meta 是单向性的,而其余的是对映性的。发现末端烷氧基链长的变化对介晶性质有显着影响。近晶A相窗口的温度范围随着烷氧基链长度的增加而变宽。化合物
-
Electrochemical sensors申请人:ISIS Innovation LTD.公开号:US10233084B2公开(公告)日:2019-03-19An electrode for use in a electrochemical sensor comprises carbon modified with a chemically sensitive redox-active compound, excluding an electrode based on carbon having derivatized thereon two redox-active species wherein at least one of said species is selected from anthraquinone, phenanthrenequinone and N,N′-diphenyl-p-phenylenediamine (DPPD). The invention further provides a pH sensor comprising: a working electrode comprising carbon modified with a chemically sensitive redox active material; and a counter electrode, wherein the ratio of the surface area of the working electrode to the surface area of the counter electrode is from 1:10 to 10:1. Also provided is a pH sensor comprising: a working electrode comprising carbon modified with a chemically sensitive redox active material, and a counter electrode, wherein the area of the working electrode is from 500 μm2 to 0.1 m2. The uses of these electrodes and sensors are also described.
-
Weygand; Gabler, Journal fur praktische Chemie (Leipzig 1954), 1940, vol. <2>155, p. 332,340作者:Weygand、GablerDOI:——日期:——
-
Burmistrov, V. A.; Kuz'mina, S. A.; Koifman, O. I., Russian Journal of Organic Chemistry, 1995, vol. 31, # 3, p. 351 - 353作者:Burmistrov, V. A.、Kuz'mina, S. A.、Koifman, O. I.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
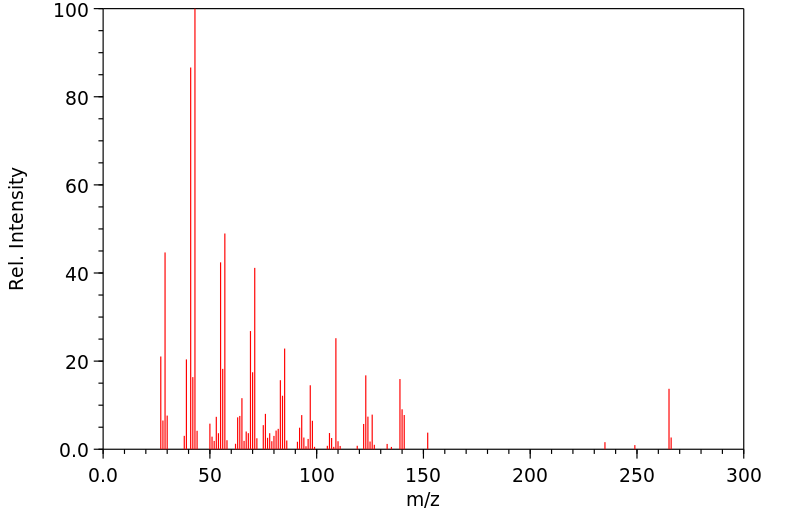
质谱MS
-
碳谱13CNMR
-
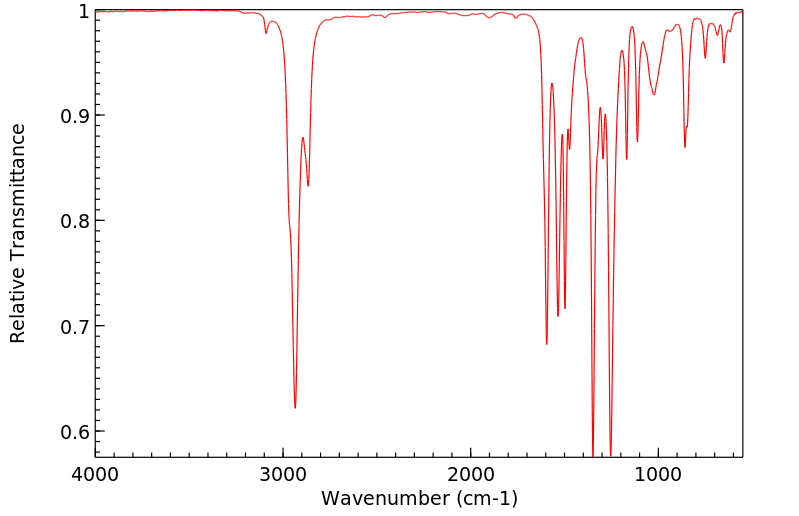
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫