trans-octahydro-1H-indene | 3296-50-2
中文名称
——
中文别名
——
英文名称
trans-octahydro-1H-indene
英文别名
trans-bicyclo<4.3.0>nonane;trans-Bicyclo<4.3.0>nonan;trans-perhydroindane;trans-hydrindane;trans-Hydrindan;(3ar,7at)-octahydroindene;(3aR,7aR)-2,3,3a,4,5,6,7,7a-octahydro-1H-indene
CAS
3296-50-2
化学式
C9H16
mdl
——
分子量
124.226
InChiKey
BNRNAKTVFSZAFA-RKDXNWHRSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-59.7°C
-
沸点:159 °C
-
密度:0.86
-
闪点:39 °C
-
大气OH速率常数:1.74e-11 cm3/molecule*sec
-
保留指数:948;949.8;955;980;950;955;964
-
稳定性/保质期:
在常温常压下稳定,其闪点为39℃,沸点为159℃。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:9
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品运输编号:UN 3295 3/PG III
-
海关编码:2902199090
SDS
反-六氢茚满
模块 1. 化学品
产品名称: trans-Hydrindane
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-六氢茚满
百分比: >96.0%(GC)
CAS编码: 3296-50-2
反-六氢茚满
模块 3. 成分/组成信息
俗名: trans-Bicyclo[4.3.0]nonane , trans-Hexahydroindane
分子式: C9H16
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
反-六氢茚满
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 159 °C
闪点: 39°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.86
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
反-六氢茚满
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: trans-Hydrindane
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-六氢茚满
百分比: >96.0%(GC)
CAS编码: 3296-50-2
反-六氢茚满
模块 3. 成分/组成信息
俗名: trans-Bicyclo[4.3.0]nonane , trans-Hexahydroindane
分子式: C9H16
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
反-六氢茚满
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 159 °C
闪点: 39°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.86
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
反-六氢茚满
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:118.熔融碳环。第十五部分。由含有正丁烯基侧链的物质合成8-甲基氢化丙烷和全氢蒽的衍生物。8-甲基氢化丙烷的脱氢摘要:DOI:10.1039/jr9380000666
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 盐酸 、 amalgamated zinc 作用下, 生成 trans-octahydro-1H-indene参考文献:名称:Hueckel et al., Justus Liebigs Annalen der Chemie, 1935, vol. 518, p. 155,173, 175摘要:DOI:
文献信息
-
Epimerization of Tertiary Carbon Centers via Reversible Radical Cleavage of Unactivated C(sp<sup>3</sup>)–H Bonds作者:Yaxin Wang、Xiafei Hu、Cristian A. Morales-Rivera、Guo-Xing Li、Xin Huang、Gang He、Peng Liu、Gong ChenDOI:10.1021/jacs.8b05753日期:2018.8.1cleavage of C(sp3)-H bonds can enable racemization or epimerization, offering a valuable tool to edit the stereochemistry of organic compounds. While epimerization reactions operating via cleavage of acidic C(sp3)-H bonds, such as the Cα-H of carbonyl compounds, have been widely used in organic synthesis and enzyme-catalyzed biosynthesis, epimerization of tertiary carbons bearing a nonacidic C(sp3)-H bondC(sp3)-H 键的可逆断裂可以实现外消旋化或差向异构化,为编辑有机化合物的立体化学提供了宝贵的工具。虽然通过裂解酸性 C(sp3)-H 键(例如羰基化合物的 Cα-H)进行的差向异构化反应已广泛用于有机合成和酶催化生物合成,但带有非酸性 C(sp3) 的叔碳的差向异构化-H 键更具挑战性,可用的实用方法很少。在这里,我们报告了第一个合成有用的协议,用于在温和条件下通过未活化的 C(sp3)-H 键与高价碘试剂苯并恶唑叠氮化物和 H2O 的可逆自由基裂解来进行叔碳差向异构化。这些反应对各种环烷烃的未活化 3° CH 键表现出优异的反应性和选择性,并为编辑传统方法难以处理的碳支架的立体化学构型提供了强大的策略。机理研究表明,N3• 作为催化氢原子穿梭的独特能力对于以高效率和选择性可逆地破坏和重组 3° CH 键至关重要。
-
Hydrogenolysis of Benzylic Alcohols on Rhodium Catalysts作者:Vidyadhar S. Ranade、Roel PrinsDOI:10.1002/(sici)1521-3765(20000117)6:2<313::aid-chem313>3.0.co;2-9日期:2000.1.17perhydroindane product by means of mass spectrometry and 13C NMR spectroscopy. The results prove that C-O bond scission takes place primarily through the direct hydrogenolysis pathway on the carbon-supported catalysts. Direct hydrogenolysis occurs on the carbon support because of the formation of a better leaving group (OH2+) from the benzylic hydroxy group and its subsequent substitution by spillover来自各种来源且具有不同铑分散体的氧化铝负载催化剂在1-茚醇的液相加氢中主要产生氢化产物perhydro-1-indanol,而碳负载催化剂主要产生CO键断裂-氢化产物过氢茚满。向反应混合物中加入有机或无机碱抑制了CO键的断裂。为了区分直接氢解或脱水然后氢化的CO键断裂的两种可能途径,已经用碳载催化剂进行了氘代研究。不仅使1-茚满醇而且使茚满和茚(CO键断裂路径中的两种可能的机理中间体)氘化。已通过质谱和13 C NMR光谱测定氘化程度和氘在所得过氢茚满产物中的掺入位置,从而获得了有关实际途径的信息。结果证明,CO键的断裂主要通过碳载催化剂上的直接氢解途径发生。由于在苄基羟基上形成了更好的离去基团(OH2 +),随后碳氢载体上发生了直接氢解,随后氢被溢出氢取代。
-
Djakowa; Losowoi, Zhurnal Obshchei Khimii, 1939, vol. 9, p. 26,31作者:Djakowa、LosowoiDOI:——日期:——
-
Selective functionalization of hydrocarbons. 6. Mechanistic and preparative studies on the regio- and stereoselective paraffin hydroxylation with peracids作者:Hans Joerg Schneider、Walter MuellerDOI:10.1021/jo00223a036日期:1985.11
-
Regioselective cyclopentane ring formation mediated by titanocene chloride作者:Pascal Rigollier、Jonathan R. Young、Lissa A. Fowley、John R. StilleDOI:10.1021/ja00181a082日期:1990.12
表征谱图
-
氢谱1HNMR
-
质谱MS
-
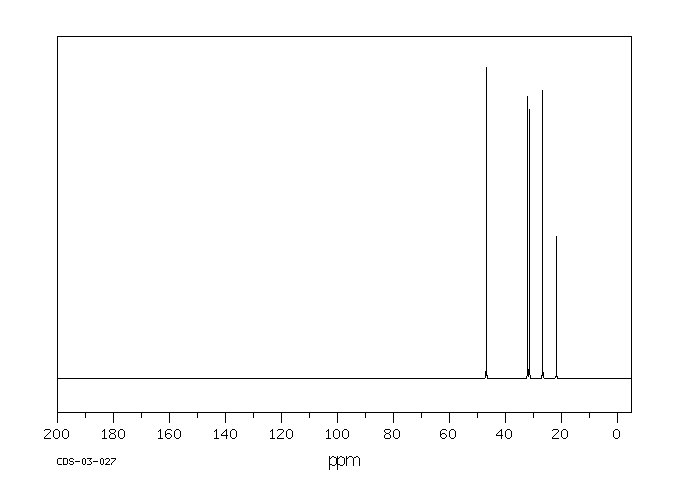
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷