1,3-二氟-2-苯基苯 | 2285-29-2
中文名称
1,3-二氟-2-苯基苯
中文别名
——
英文名称
2,6-difluorobiphenyl
英文别名
2,6-difluoro-1,1'-biphenyl;1,3-difluoro-2-phenylbenzene
CAS
2285-29-2
化学式
C12H8F2
mdl
——
分子量
190.192
InChiKey
WTUKEAUROZESDP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2903999090
SDS
反应信息
-
作为反应物:描述:1,3-二氟-2-苯基苯 以69%的产率得到1-[(2,6-Difluoro-3-phenyl)phenyl]cyclopentanol参考文献:名称:Tetracyclic compound摘要:这项发明涉及一种由化学式(I)表示的新型四环化合物:##STR1##或其药理学上可接受的盐。本发明的化合物具有强烈的免疫抑制活性,并可用作免疫抑制剂以及治疗自身免疫疾病的药物。公开号:US05646283A1
-
作为产物:描述:参考文献:名称:金催化的碘芳烃脱羧交叉偶联反应摘要:该报告详细介绍了(杂)芳基羧酸酯与碘芳烃在金催化剂存在下的脱羧交叉偶联(> 25 个例子,产率高达 96%)。该反应是位点特异性的,它克服了与金催化氧化偶联反应相关的先前限制。(杂)芳基羧酸酯的反应性与场效应参数(Fortho)定性相关。与分离的金配合物的反应和 DFT 计算支持通过在金 (I) 阳离子上氧化加成进行的机制,脱羧在金 (I) 或银 (I) 物种中都是可行的。DOI:10.1021/jacs.0c06244
文献信息
-
Flash Generation of a Highly Reactive Pd Catalyst for Suzuki-Miyaura Coupling by Using a Flow Microreactor作者:Aiichiro Nagaki、Naofumi Takabayashi、Yuya Moriwaki、Jun-ichi YoshidaDOI:10.1002/chem.201201579日期:2012.9.17Superflash! Flash chemistry enables the use of highly reactive unstable species as catalysts for chemical synthesis. Fast micromixing of a solution of [Pd(OAc)2] and that of tBu3P in 1:1 mole ratio gave a solution of a highly reactive unstable species, which was immediately transferred to a vessel by using a flow microreactor, in which Suzuki–Miyaura coupling was conducted (see scheme).
-
Reactions of Dry Arenediazoniumo-Benzenedisulfonimides with Triorganoindium Compounds作者:Margherita Barbero、Silvano Cadamuro、Stefano Dughera、Cinzia GiavenoDOI:10.1002/ejoc.200600527日期:2006.11The reaction between various arenediazonium o-benzenedisulfonimides and triorganoindium compounds is described. Depending on the reaction conditions, it is possible to obtain biaryls (16 examples, average yield of 79 %) or diaryldiazenes (18 examples, average yield of 81 %). o-Benzenedisulfonimide can be recovered and reused to prepare additional arenediazonium o-benzenedisulfonimides. (© Wiley-VCH
-
Continuous-Flow Synthesis of Biaryls by Negishi Cross-Coupling of Fluoro- and Trifluoromethyl-Substituted (Hetero)arenes作者:Stefan Roesner、Stephen L. BuchwaldDOI:10.1002/anie.201605584日期:2016.8.22continuous‐flow method for the regioselective arylation of fluoroarenes and fluoropyridines has been developed. The telescoped procedure reported here consists of a three‐step metalation, zincation, and Negishi cross‐coupling sequence, providing efficient access to a variety of functionalized 2‐fluorobiaryl products. Precise temperature control of the metalation step, made possible by continuous‐flow technology
-
[EN] COMPLEXES AND CATALYTIC PROCESSES<br/>[FR] COMPLEXES ET PROCÉDÉS CATALYTIQUES申请人:UNIV SUSSEX公开号:WO2015087062A1公开(公告)日:2015-06-18The present application is directed towards complexes of formula (I), to methods for preparing such complexes, and to use of such complexes in catalysis. The complexes show utility in a range of catalytic cycles, including Pd(ll)/Pd(IV) cycles. (Formula (I))本申请涉及公式(I)的配合物,以及制备这种配合物的方法,以及在催化中使用这种配合物。这些配合物在一系列催化循环中显示出实用性,包括Pd(II)/Pd(IV)循环。 (公式(I))
-
General Method for Functionalized Polyaryl Synthesis via Aryne Intermediates作者:Thanh Truong、Milad Mesgar、Ky Khac Anh Le、Olafs DaugulisDOI:10.1021/ja504886x日期:2014.6.18complements transition-metal-catalyzed direct arylation and allows access to structures that are not easily accessible via other direct arylation methods. The reactions are highly functional-group tolerant, with alkene, ether, dimethylamino, trifluoromethyl, ester, cyano, halide, hydroxyl, and silyl functionalities compatible with reaction conditions.
表征谱图
-
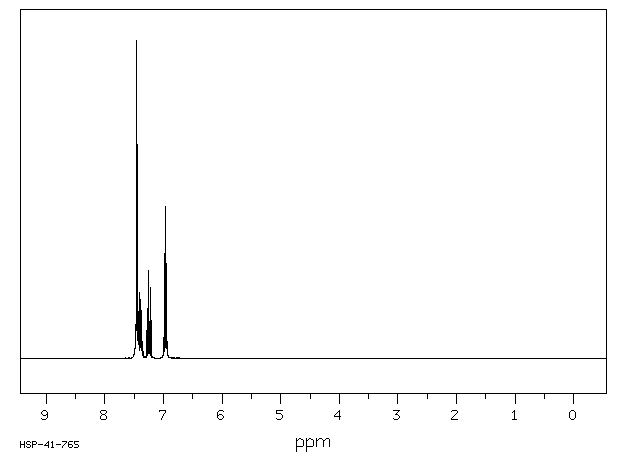
氢谱1HNMR
-
质谱MS
-
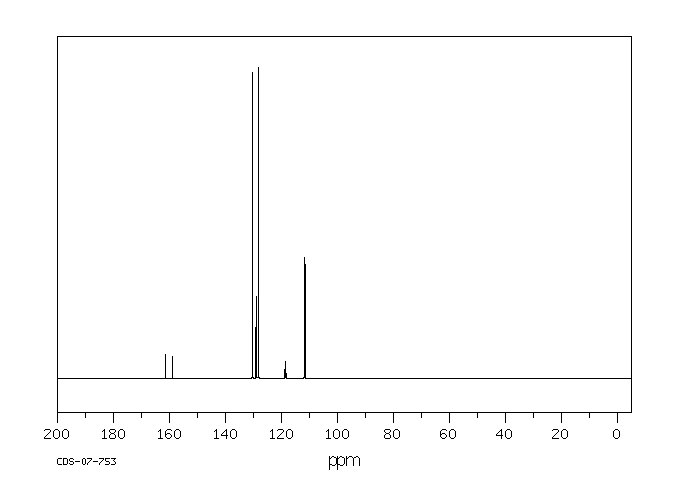
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫