2-氯苯甲酸酐 | 49619-43-4
中文名称
2-氯苯甲酸酐
中文别名
——
英文名称
2-chlorobenzoic anhydride
英文别名
o-chlorobenzoic anhydride;2-chloro-benzoic acid-anhydride;2-Chlor-benzoesaeure-anhydrid;chlorobenzoic anhydride;2-Chlorbenzoesaeure-anhydrid;2-Chlorbenzoesaeureanhydrid;(2-chlorobenzoyl) 2-chlorobenzoate
CAS
49619-43-4
化学式
C14H8Cl2O3
mdl
——
分子量
295.122
InChiKey
OLENANGIJWDOPG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:对甲苯磺酰氯实际合成羧酸衍生物的研究摘要:背景:羧酸衍生物是公认的重要一类试剂,经常用于制备各种精细或特殊化学物质,例如酰胺,酯,肽,药物和染料。尽管开发了几种制备这些化合物的方法,但其中许多方法都存在困难,包括收率低,反应温度高,反应条件苛刻,后处理繁琐以及不适合规模化使用。 方法:在超声辐射和常规条件下,在K2CO3和乙腈为溶剂的情况下,通过羧酸与TsCl的反应开发羧酸酐的合成方法。另外,在所产生的羧酸酐存在下和在相同条件下加入叠氮化钠的情况下,一锅合成酰基叠氮化物。 结果:在超声辐射和常规搅拌下,使用TsCl合成了一系列羧酸酐和酰基叠氮化物,其操作简单,反应条件温和,收率高,放大能力不受任何限制。在大多数情况下,与常规方法相比,超声辐射下的反应在收率和反应时间上都更好。 结论:已经开发了一种在超声辐射和常规搅拌下制备羧酸酐和酰基叠氮化物的简便方法。对于以前的报告,本方法是实用且高效的替代方法。该方法的主要优点是:(i)工艺简DOI:10.2174/1570178614666170329153759
-
作为产物:参考文献:名称:羧酸与羧酸与Ph 3 P / I 2 / Et 3 N反应生成的酸酐和意外的N,N-二乙酰胺摘要:研究了由磷介导的羧酸活化作用形成酸酐。在各种体系下,取决于试剂的反应性,在碱的存在下苯甲酸的活化导致苯甲酸酐以不同的速率形成。使用Ph 3 P–I 2 / Et 3 N组合,大多数芳酸在5–10分钟内即可高产率转化为相应的酸酐。然而,对于硝基取代的衍生物,出乎意料的是,N,N分离了-二乙酰胺而没有酸酐形成。这些结果表明取代基在控制这些潜在的副反应中具有显着的作用,其可显着影响由phospho物种促进的酰化反应的产率。DOI:10.1016/j.tetlet.2015.12.009
文献信息
-
Synthesis of symmetric anhydrides using visible light-mediated photoredox catalysis作者:Marlena D. Konieczynska、Chunhui Dai、Corey R. J. StephensonDOI:10.1039/c2ob25463h日期:——A new approach to anhydride formation is reported via activation of C–O bonds by the Vilsmeier–Haack reagent formed by Ru(bpy)3Cl2 and CBr4 in DMF. Various aryl and alkyl carboxylic acids are converted to the corresponding anhydrides in excellent yields.
-
Facile Synthesis of CarboxylicAnhydrides Using 4,5-Dichloro-2-[(4-nitrophenyl)sulfonyl]pyridazin-3(2<i>H</i>)-one作者:Yong-Jin Yoon、Jeum-Jong Kim、Yong-Dae Park、Woo Song Lee、Su-Dong ChoDOI:10.1055/s-2003-40512日期:——A novel and facile synthesis of carboxylic anhydrides from carboxylic acid using 4,5-dichloro-2-[(4-nitrophenyl)sulfonyl[pyridazin-3(2H)-one (2) is presented. Treatment of aliphatic or aromatic carboxylic acids with 2 in the presence of base in organic solvents gave the corresponding anhydrides in good or excellent yields.
-
Ligand-Free Palladium-Catalyzed Aerobic Oxidative Coupling of Carboxylic Anhydrides with Arylboronic Acids作者:Weiyan Yin、Haifeng He、Yani Zhang、Tong LongDOI:10.1002/asia.201402314日期:2014.9We report a new, effective and environmentally friendly protocol for selective aerobic oxidative coupling of arylboronic acids with carboxylic anhydrides in the presence of ligand‐free palladium catalyst. The aryl benzoates are obtained in good to excellent yields.
-
Visible-Light-Mediated Photocatalytic Difunctionalization of Olefins by Radical Acylarylation and Tandem Acylation/Semipinacol Rearrangement作者:Giulia Bergonzini、Carlo Cassani、Haldor Lorimer-Olsson、Johanna Hörberg、Carl-Johan WallentinDOI:10.1002/chem.201504985日期:2016.3.1A novel method for the mild photoredox‐mediated tandem radical acylarylation and tandem acylation/semipinacol rearrangement has been developed. The synthesis of highly functionalized ketones bearing all‐carbon α‐ or β‐quaternary centers has been achieved using easily available symmetric aromatic carboxylic anhydrides as the acyl radical source. The method allows for a straightforward introduction of开发了一种轻度的光氧化还原介导的串联自由基酰基化和串联酰化/ semipinacol重排的新方法。使用容易获得的对称芳族羧酸酐作为酰基自由基源,可以合成带有全碳α-或β-季中心的高度官能化的酮。该方法允许在单个操作中直接引入酮基官能团并伴随分子复杂性的构建。
-
One-pot synthesis of thioesters with sodium thiosulfate as a sulfur surrogate under transition metal-free conditions作者:Yen-Sen Liao、Chien-Fu LiangDOI:10.1039/c8ob00178b日期:——In this paper, we report an efficient synthetic method for thioester formation from sodium thiosulfate pentahydrate, organic halides, and aryl anhydrides. In the one-pot two-step reactions developed in this study, sodium thiosulfate was used as the sulfur surrogate for acylation with anhydrides, followed by substitution with organic halides through the in situ generation of thioaroylate. Furthermore
表征谱图
-
氢谱1HNMR
-
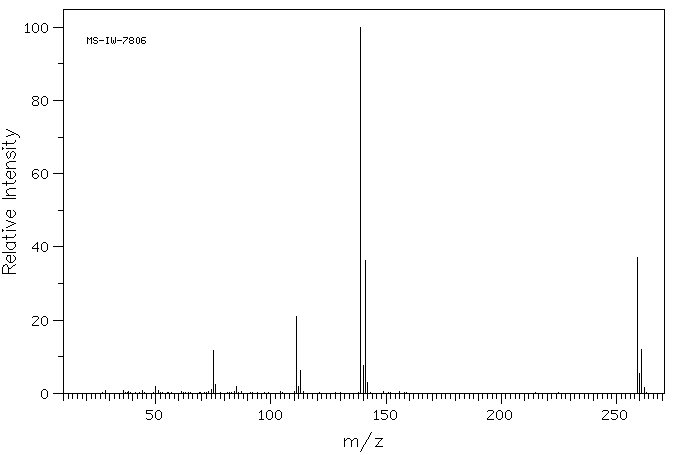
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫