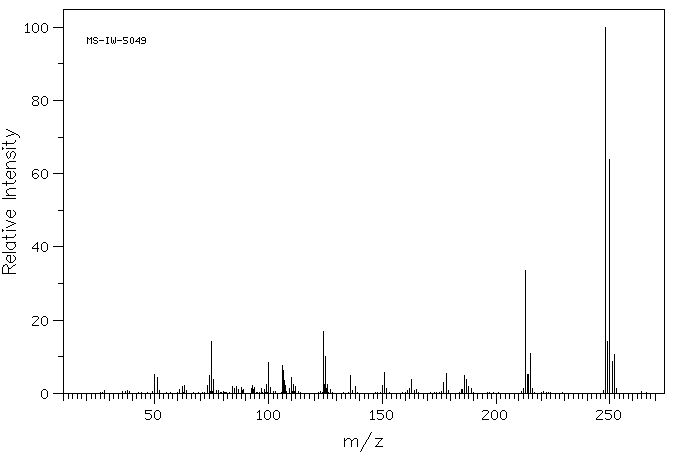
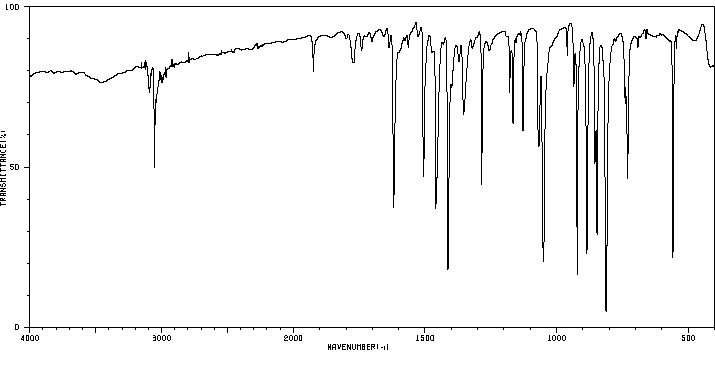
2,7-二氯吩嗪 | 3372-79-0
中文名称
2,7-二氯吩嗪
中文别名
——
英文名称
2,7-dichloro-phenazine
英文别名
2,7-Dichlor-phenazin;2,7-Dichlorophenazine
CAS
3372-79-0
化学式
C12H6Cl2N2
mdl
——
分子量
249.099
InChiKey
NWAQHIWGPITNGK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:266-268 °C(Solv: benzene (71-43-2))
-
沸点:420.9±15.0 °C(Predicted)
-
密度:1.482±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Lehn, Jean-Marie; Schmidt, Frederic; Vigneron, Jean-Pierre, Journal of Heterocyclic Chemistry, 1990, vol. 27, # 6, p. 1633 - 1637摘要:DOI:
-
作为产物:描述:参考文献:名称:The Halogen-activating Effect of the N-Oxide Group in Chlorophenazine-N-oxides. Synthesis of an Isomer of Iodinin摘要:DOI:10.1021/ja01145a146
文献信息
-
Super electron donor-mediated reductive transformation of nitrobenzenes: a novel strategy to synthesize azobenzenes and phenazines作者:Kanako Nozawa-Kumada、Erina Abe、Shungo Ito、Masanori Shigeno、Yoshinori KondoDOI:10.1039/c8ob00271a日期:——The transformation of nitrobenzenes into azobenzenes by pyridine-derived super electron donor 2 is described. This method provides an efficient synthesis of azobenzenes because of not requiring the use of expensive transition-metals, toxic or flammable reagents, or harsh conditions. Moreover, when using 2-fluoronitrobenzenes as substrates, phenazines were found to be obtained. The process affords a
-
Cyclointercalands.- Incorporation of the phenazine group and of metal binding subunits into macrocyclic receptor molecules.作者:Jean-Marie Lehn、Frédéric Schmidt、Jean-Pierre VigneronDOI:10.1016/s0040-4039(00)80730-3日期:1988.1Macrocyclic receptor molecules containing two phenazine groups 1, or combining a phenazine unit and a metal ion binding site, 2 and 3, have been synthesized; evidence has been obtained for the binding of anionic substrate to the bis-N-methylated 1-Me22+ and of metal ions (Ag+, Cu+) to 2 and 3.
-
Boron trifluoride promoted reaction of benzenesulphenanilides with alkenes作者:Luisa Benati、P.Carlo Montevecchi、Piero SpagnoloDOI:10.1016/s0040-4020(01)87520-7日期:1986.1The boron trifluoride-promoted reaction of a series of 3'- and 4'-substituted benzenesulphenanilides (1) with various alkenes has been investigated as a potential synthetic route to arylaminosulphides. The benzenesulphenanilides (1) investigated generally afford arylamino-sulphenylation adducts in fair to good yields except for the methoxy-substituted anilides (le and f), which largely lead to decomposition
-
Reactivity and substituent effects in the cyclization of N -aryl-2-nitrosoanilines to phenazines作者:Zbigniew Wróbel、Karolina Plichta、Andrzej KwastDOI:10.1016/j.tet.2017.04.046日期:2017.6estimated on the base of the observed reaction times. A strong opposite effect of substituents located at position para to the nitroso group and those located para to the amino group in the side ring was observed. Mechanistic explanation, based on the electronic properties of the substituents and their mesomeric effects, was presented. The usefulness of the obtained data for the designed syntheses of
-
2-Nitroso-<i>N</i>-arylanilines: Products of Acid-Promoted Transformation of σ<sup>H</sup> Adducts of Arylamines and Nitroarenes作者:Zbigniew Wróbel、Andrzej KwastDOI:10.1055/s-2007-982534日期:2007.6Anions generated from arylamines react with substituted nitrobenzenes to form ÏH adducts, which, under protonation with acetic acid, undergo transformation to 2-nitroso-N-arylamines, susceptible to reduction, condensation and cyclization reactions.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮