3,3',4,4'-四甲基联苯 | 4920-95-0
中文名称
3,3',4,4'-四甲基联苯
中文别名
3,3",4,4"-四甲基联苯
英文名称
3,4,3',4'-Tetramethylbiphenyl
英文别名
3,3',4,4'-tetramethylbiphenyl;3,3',4,4'-tetramethyl-1,1'-biphenyl;4-(3,4-dimethylphenyl)-1,2-dimethylbenzene
CAS
4920-95-0
化学式
C16H18
mdl
MFCD00130219
分子量
210.319
InChiKey
YXBIAYXZUDJVEB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74-77°C
-
沸点:317.5±37.0 °C(Predicted)
-
密度:0.956
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
安全说明:S22,S24/25
-
海关编码:2902909090
-
储存条件:常温、避光、存放在阴凉干燥处并密封保存。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,4-四甲基-5-苯基苯 3,4,5,6-tetramethylbiphenyl 30581-98-7 C16H18 210.319 1-溴-2-(2-溴-4,5-二甲基苯基)-4,5-二甲基苯 2,2'-dibromo-4,4',5,5'-tetramethylbiphenyl 211434-29-6 C16H16Br2 368.111
反应信息
-
作为反应物:描述:参考文献:名称:一种3,3',4,4'-联苯四甲酸二酐的合成方法摘要:本发明公开了一种3,3',4,4'‑联苯四甲酸二酐的合成方法,属于化学合成领域。本发明先将4‑溴邻二甲苯加入镁粉和碘单质制备得到反应物,将反应物和4‑氯邻二甲苯在乙酰丙酮镍作为催化剂的条件下加热反应,得到3,3’,4,4’‑四甲基联苯,氧化、酸化后得3,3’,4,4’‑联苯四甲酸,最后真空高温脱水,即可得到3,3',4,4'‑联苯四甲酸二酐。公开号:CN106518820A
-
作为产物:描述:3,4-二甲基苯硼酸 在 air 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 16.0h, 以93%的产率得到3,3',4,4'-四甲基联苯参考文献:名称:Aerobic homocoupling of arylboronic acids catalysed by copper terephthalate metal–organic frameworks摘要:铜对苯二甲酸MOF被用作一种环保、高效且可重复使用的非均相催化剂,以在温和反应条件下进行芳基硼酸的有氧同偶联,产出相应的对称联苯。该方法能够耐受芳基硼酸中存在的各种取代基,如卤素、氰基和硝基。催化性能已与其他铜基MOFs进行比较,如MOF-101、[Cu(pdc)2]NH2Me2、[Cu2(ndc)2ted]n和[Cu(H2L)]n,以及其他铜盐催化剂。Sheldon测试确认了催化剂的非均相特性,在优化条件下可以重复使用,仅有轻微的活性损失。还提出了同偶联反应的机制。催化剂制备的简单性、稳定性、底物选择性、易于回收和再生使得该催化系统在多种催化反应和工业过程中具有潜在的应用价值。DOI:10.1039/c4gc00056k
文献信息
-
Chromium(II)-Catalyzed Diastereoselective and Chemoselective Csp<sup>2</sup>–Csp<sup>3</sup> Cross-Couplings Using Organomagnesium Reagents作者:Jie Li、Qianyi Ren、Xinyi Cheng、Konstantin Karaghiosoff、Paul KnochelDOI:10.1021/jacs.9b08586日期:2019.11.13A simple protocol for performing chromium-catalyzed highly diastereoselective alkylations of arylmagnesium halides with cyclohexyl iodides at ambient temperature has been developed. Furthermore, this ligand-free CrCl2 enables efficient electrophilic alkenylations of primary, secondary and tetiary alkylmagnesium halides with readily available alkenyl acetates. Moreover, this chemoselective C‒C coupling
-
METHOD FOR PRODUCING MULTISUBSTITUTED BIPHENYL COMPOUND AND SOLID CATALYST TO BE USED THEREIN申请人:KYUSHU UNIVERSITY, NATIONAL UNIVERSITY CORPORATION公开号:US20150274689A1公开(公告)日:2015-10-01A method for producing a multisubstituted biphenyl compound is represented by the following formula (2), including a step of coupling a substituted benzene compound represented by the following formula (1) in the presence of a solid catalyst with gold immobilized onto a support.
-
2,2`-二(三氟甲基)-4,4`,5,5`-联苯二酐的制备方法申请人:江苏尚莱特医药化工材料有限公司公开号:CN106699709A公开(公告)日:2017-05-24
-
Homocoupling of Iodoarenes and Bromoalkanes Using Photoredox Gold Catalysis: A Light Enabled Au(III) Reductive Elimination作者:Huy Tran、Terry McCallum、Mathieu Morin、Louis BarriaultDOI:10.1021/acs.orglett.6b02021日期:2016.9.2formation of homocoupled alkane byproducts have been identified in the reduction of bromoalkanes via photoredox gold catalysis with dimeric Au(I) complexes. This prompted further investigation into the mechanism of formation of these byproducts and the diversity of C–X bonds amenable to this transformation. Examples were found when considering bromoalkanes while a wide variety of iodoarenes underwent this
-
Iridium-Promoted, Palladium-Catalyzed Direct Arylation of Unactivated Arenes作者:Landon J. Durak、Jared C. LewisDOI:10.1021/om401221v日期:2014.2.10Examining the scope of this reaction led to the discovery that Cp*(PMe3)IrMeCl activates C–H bonds on arene substrates that undergo subsequent Pd-catalyzed cross-coupling with aryl iodides. This Ir-promoted, Pd-catalyzed direct arylation is notable for its distal selectivity on substituted arenes lacking directing groups or a particular electronic bias.
表征谱图
-
氢谱1HNMR
-
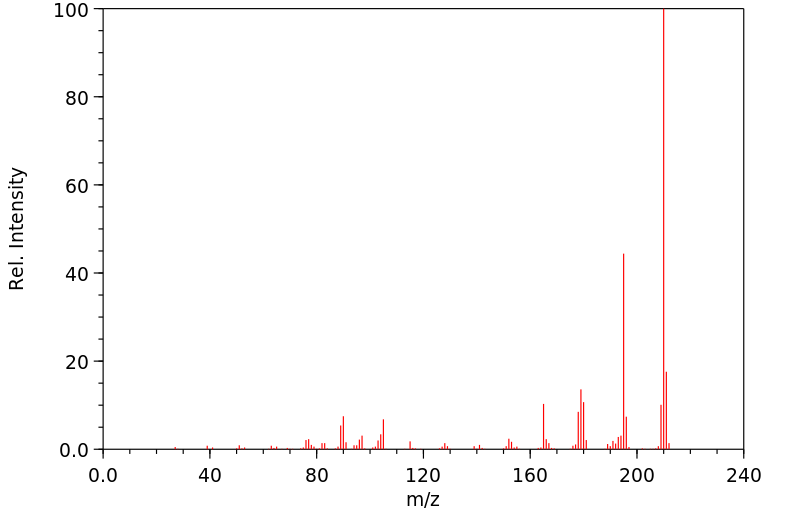
质谱MS
-
碳谱13CNMR
-
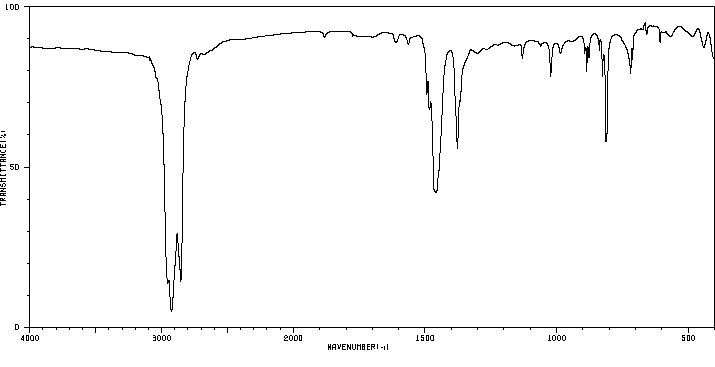
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫